Filter results
You can narrow down the results using the filters
Audience
Publication type
Topics
Our work
Diseases
Year
22 results
-

Draft Cost Recovery Implementation Statement – Administration of the Prescribed List of Medical Devices and Human Tissue Products
This draft cost recovery implementation statement describes how the Department of Health and Aged Care recovers the costs of administering the Prescribed List from 1 July 2025 to 30 June 2026. -

ATAGI statement on the administration of COVID-19 vaccines in 2025
Australian Technical Advisory Group on Immunisation (ATAGI) advice for immunisation providers regarding the administration of COVID-19 vaccines in 2025. -
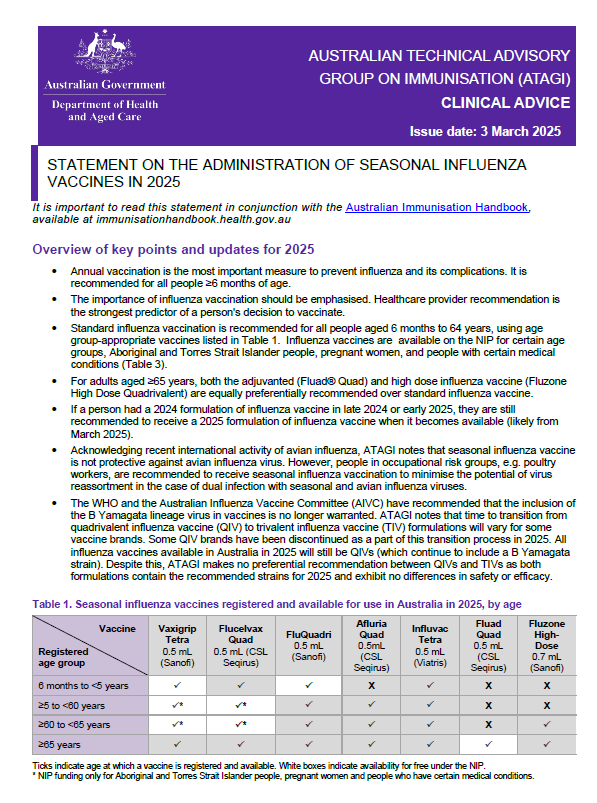
ATAGI statement on the administration of seasonal influenza vaccines in 2025
Australian Technical Advisory Group on Immunisation (ATAGI) advice regarding the administration of 2025 seasonal influenza vaccines. -

Update – Extended timeframe for release of Consultation Paper 2
This update provides information on the progress of Consultation Paper 2 and upcoming engagements with stakeholders. -

National Women’s Health Advisory Council Statement
The National Women’s Health Advisory Council strongly commend the Australian Government’s significant investment made to improve women’s health in Australia. -

Budget 2024–25: Health Portfolio Additional Estimates Statements
Portfolio Additional Estimates Statements (PAES) focus on explaining changes to the proposed allocation of resources by outcome since the Budget. The PAES provides information on new measures and their impact on the financial and/or non-financial planned performance of programs. -

National Sport Strategy – Sport Ministers communique
Australia’s Sport Ministers welcome the launch of the Australian Government’s new National Sport Strategy: Sport Horizon, which was developed in collaboration with states and territories. -

ATAGI Clinical Guidance on the use of vaccines for the prevention of Mpox
Australian Technical Advisory Group on Immunisation (ATAGI) clinical guidance on the use of vaccines for the prevention of Mpox in 2024. -

Statement of Intent from the National Rural Health Commissioner – 2 September 2024 to 30 June 2026
The Statement of Intent outlines the National Rural Health Commissioner’s priorities and approach to achieving the obligations referred to in the Assistant Minister’s Statement of Expectations. -

National Joint Replacement Registry Cost Recovery Implementation Statement
This statement describes how the Department of Health and Aged Care recovers the costs of administering the National Joint Replacement Registry. -

NRAS Complexity Review Update – Completion of phase 3
The Review Team has appreciated the input and feedback on Consultation Paper 1, received in written submissions and through the policy design forums that have been held nationwide. -

Australian Technical Advisory Group on Immunisation (ATAGI) Annual Statement on Immunisation 2024
The ATAGI 2024 Annual Statement on Immunisation is the fourth publication in this series. The statement highlights the key successes, trends and challenges in the use of vaccines and control of vaccine preventable diseases in Australia in 2023, and includes advice to address key issues for 2024. -

ATAGI statement on the transition from quadrivalent to trivalent seasonal influenza vaccines in Australia
Australian Technical Advisory Group on Immunisation (ATAGI) statement about the transition from quadrivalent to trivalent seasonal influenza vaccines in Australia. -

Statement from Australian Health Ministers: Analysis of unmet need for psychosocial supports outside of the National Disability Insurance Scheme
This statement from Australian Health Ministers provides key context for the analysis of unmet need for psychosocial supports outside of the National Disability Insurance Scheme, key findings and limitations and next steps for governments’ considerations. -

NRAS Complexity Review update – 18 June 2024
Sue Dawson outlines next steps in the review process and seeks stakeholder advice on how their jurisdiction would prefer to participate and connect throughout the review. -

ATAGI statement on the administration of COVID-19 vaccines in 2024
Australian Technical Advisory Group on Immunisation (ATAGI) advice for immunisation providers regarding the administration of COVID-19 vaccines in 2024. -
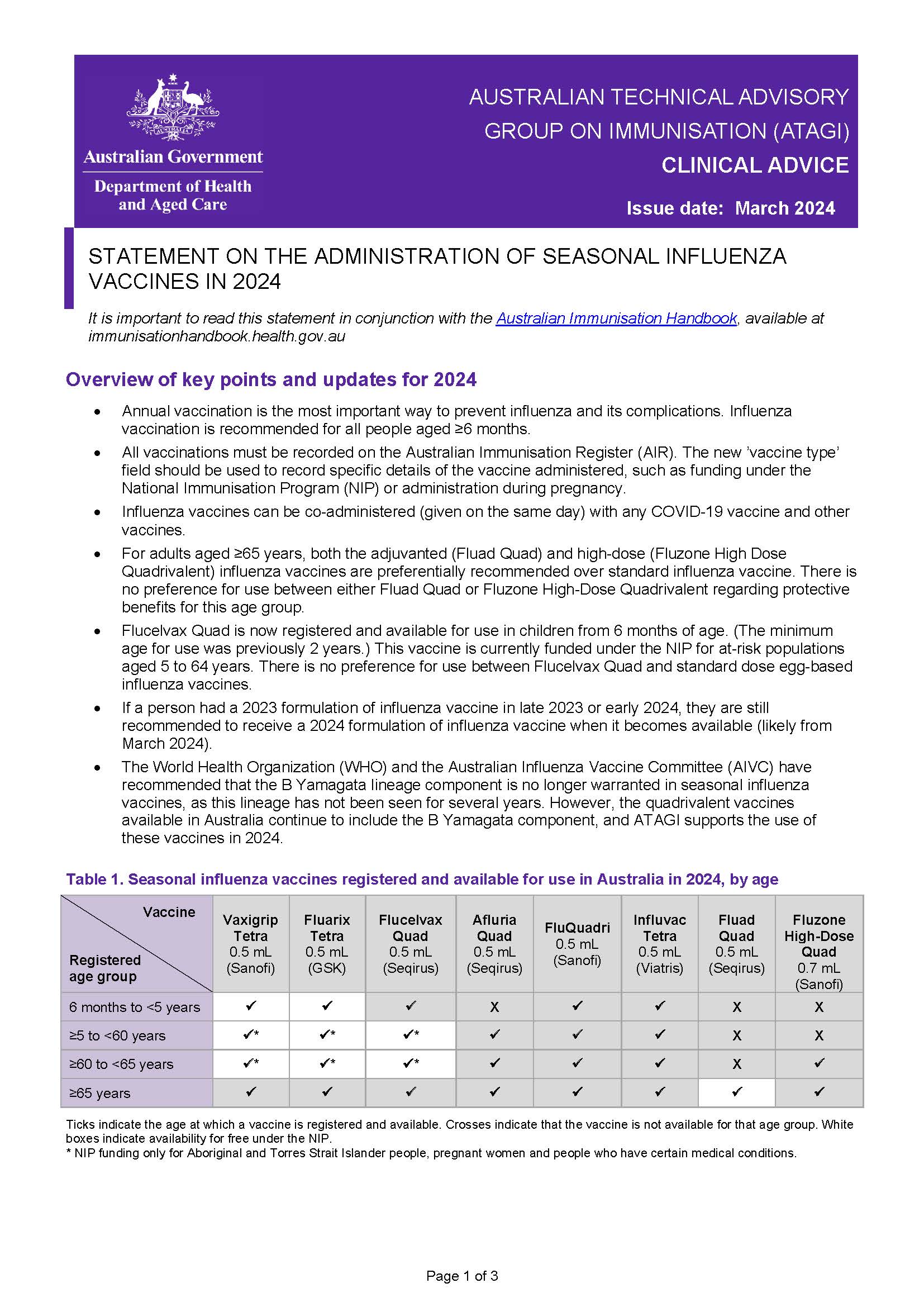
ATAGI statement on the administration of seasonal influenza vaccines in 2024
Australian Technical Advisory Group on Immunisation (ATAGI) advice regarding the administration of 2024 seasonal influenza vaccines. -

Australian Technical Advisory Group on Immunisation (ATAGI) Annual Statement on Immunisation 2023
The ATAGI 2023 Annual Statement on Immunisation is the third publication in this series. It covers key successes, trends and challenges in the use of vaccines and control of vaccine-preventable diseases in 2023. It also signals ATAGI’s priority actions for addressing key issues for 2023. -

ATAGI statement on the intradermal use of Imojev Japanese encephalitis vaccine
ATAGI has reviewed the evidence on the intradermal (ID) administration of Japanese encephalitis (JE) vaccines and considered the potential use of Imojev as a dose-sparing mechanism, in the context of the risk of future outbreaks, expanded eligibility in risk areas, and ongoing supply considerations. -

Australian Technical Advisory Group on Immunisation (ATAGI) Annual Statement on Immunisation 2022
The ATAGI 2022 Annual Statement on Immunisation is the second publication in this series, delayed due to COVID-19. It covers key successes, trends and challenges in the use of vaccines and control of vaccine-preventable diseases in 2021. -
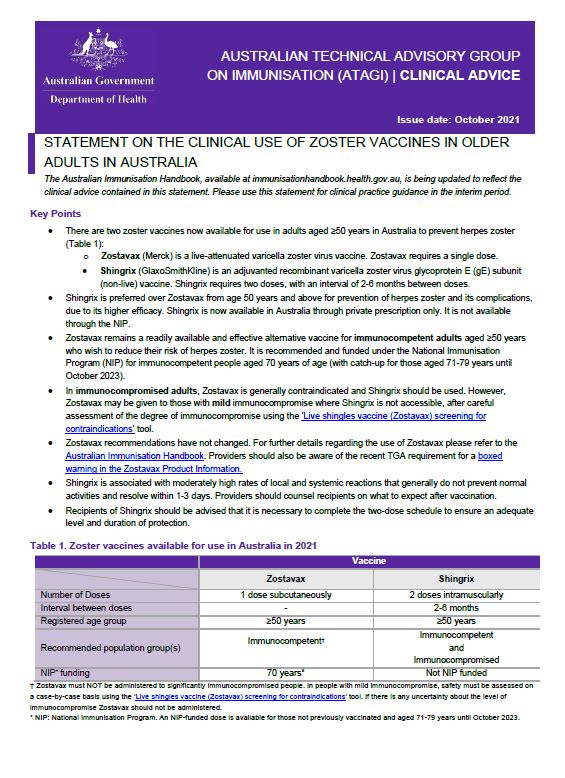
Statement on the clinical use of zoster vaccine in older adults in Australia
This ATAGI clinical statement provides recommendations for the zoster vaccine and Shingrix vaccine. -

Australian Technical Advisory Group on Immunisation (ATAGI) Annual Statement on Immunisation 2021
The ATAGI 2021 Annual Statement on Immunisation is the first publication in this series. It covers key successes, trends and challenges in the use of vaccines and control of vaccine-preventable diseases in 2020. It also signals ATAGI’s priority actions for addressing key issues for 2021 and beyond.
