Filter results
You can narrow down the results using the filters
Audience
Topics
Our work
Year
326 results
-
Toolkits to promote cervical screening
Help raise awareness about cervical screening and the Cervical Screening Test self-collection option. -
Training for upcoming changes to clinical guidelines for cervical screening
Healthcare providers, make sure you’re up to date on upcoming changes to the National Cervical Screening Program clinical guidelines. -
General practices: Help us promote our life-saving cancer screening programs
We have sent cancer screening packs to more than 7,000 general practices, and are seeking your help to promote our life-saving cancer screening programs. -
Update on Pathology Accreditation Standards
Important information for healthcare providers and pathology laboratories regarding changes to the ‘Requirements for Cervical Screening (First edition 2023)’ from 1 August 2024. -
Webinars on cervical screening for Aboriginal and/or Torres Strait Islander patients
NACCHO are hosting two CPD accredited webinars for healthcare workers on the cervical screening self-collection option. The webinars will focus on how to support Aboriginal and/or Torres Strait Islander women and people with a cervix in their cervical screening. -
Equity in cervical screening webinar
Healthcare providers are invited to attend a webinar on best practice cervical screening for people with disability. -
Cervical screening self-collection
Healthcare practices and clinics offering cervical screening are encouraged to set themselves up to support patient screening choice. -
Protecting older people in the festive season
Christmas, summer and the festive season is a time many of us spend with our family and friends. Make sure you know the basics and protect yourself, family and friends from COVID-19. -
ATAGI recommendations on use of the Moderna and Pfizer monovalent Omicron XBB.1.5 COVID-19 vaccines
Recommendations from the Australian Technical Advisory Group on Immunisation (ATAGI) on the use of XBB 1.5 COVID-19 vaccines. -
Eliminating Cervical Cancer in Australia
Australia now has a national strategy to help us achieve our goal of eliminating cervical cancer by 2035 – the first country in the world to do so. -
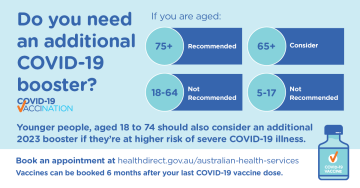
ATAGI update additional 2023 COVID-19 vaccination dose
The Australian Technical Advisory Group on Immunisation (ATAGI) recently updated their advice for the COVID-19 booster dose. -
ATAGI Update on the COVID-19 Vaccination Program
Recommendations from the Australian Technical Advisory Group on Immunisation (ATAGI) regarding an additional COVID-19 dose for highest risk people in 2023. These recommendations are in addition to the previous ATAGI COVID-19 vaccine booster advice published in February 2023. -
Updated cervical screening education course
Healthcare providers – build knowledge and skills to offer your patients the self-collection option for cervical screening HPV testing. -
ATAGI advice on the preferential use of bivalent COVID-19 vaccines for primary vaccination of people aged 12 years or older
ATAGI has made recommendations on the use of bivalent COVID-19 vaccines as a primary course. -
Top up your COVID-19 protection
The Australian Government has launched a new advertising campaign to encourage adults to get a COVID-19 booster if it has been 6 months or more since their last COVID-19 vaccination or infection. -
Early cancer detection and screening for Aboriginal and Torres Strait Islander people
It’s easy to forget about cancer screening. But detecting cancer early saves lives. Learn more about cancer screening programs and how to enter the Beautiful Shawl competition. -
Release of the Australian Government's response to the Review of COVID-19 Vaccine and Treatment Purchasing and Procurement
The Australian Government has responded to the Halton review into COVID-19 vaccine and treatment procurement. -
ATAGI recommendations on use of the Moderna bivalent (Original/Omicron BA.4/5) COVID-19 vaccine
Recommendations from the Australian Technical Advisory Group on Immunisation (ATAGI) on the use of the Moderna bivalent (Original/Omicron BA.4/5) COVID-19 vaccine. -
ATAGI recommendations on use of the Pfizer bivalent (Original/Omicron BA.4/5) COVID-19 vaccine
Recommendations from the Australian Technical Advisory Group on Immunisation (ATAGI) on the use of the Pfizer bivalent (Original/Omicron BA.4/5) COVID-19 vaccine. -
Travellers from China required to undertake COVID-19 testing before travel
The Australian Government will introduce pre-departure testing for COVID-19 for people travelling to Australia from the People’s Republic of China. -
ATAGI recommendations on use of the Pfizer COVID-19 vaccine for children aged 6 months to 4 years
Recommendations from the Australian Technical Advisory Group on Immunisation (ATAGI) on the use of the Pfizer COVID-19 vaccine for children aged 6 months to 4 years (Pfizer 6 months to 4 years). -
ATAGI recommendations on use of the Pfizer bivalent (Original/Omicron BA.1) COVID-19 vaccine
Recommendations from the Australian Technical Advisory Group on Immunisation (ATAGI) the Pfizer bivalent (Original/Omicron BA.1) COVID-19 vaccine. -
ATAGI update on boosters following COVID-19 meeting on 11 November 2022
An update from the Australian Technical Advisory Group on Immunisation (ATAGI) following their meeting on 11 November 2022. -
New COVID-19 variant leads to increase in cases
A statement from Professor Paul Kelly, Australian Government Chief Medical Officer, on the increase in COVID-19 cases due to a new Omicron variant. -
ATAGI recommendations for a booster dose of the paediatric Pfizer COVID-19 vaccine in children aged 5 to 11 years
Recommendations from the Australian Technical Advisory Group on Immunisation (ATAGI) on Pfizer booster doses for children aged 5 to 11 years.