Filter results
You can narrow down the results using the filters
Audience
Topics
Our work
Year
70 results
-

Consultation Paper 10 – General Use Items utilisation, expenditure and integrity
This circular is to advise stakeholders of the publication of a high level summary and individual submissions. -
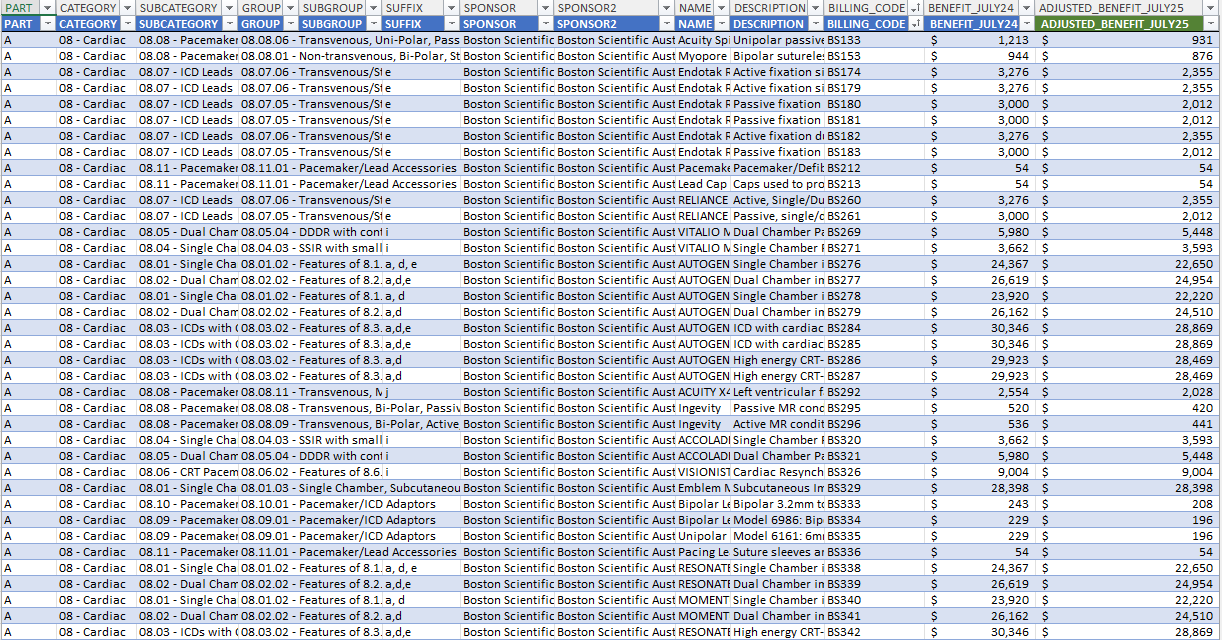
Advice on the benefit reductions to Cardiac Implantable Electronic Devices
The document contains advice on the third and final reduction of benefits for Cardiac Implantable Electronic Devices (CIED) effective 1 July 2025. -
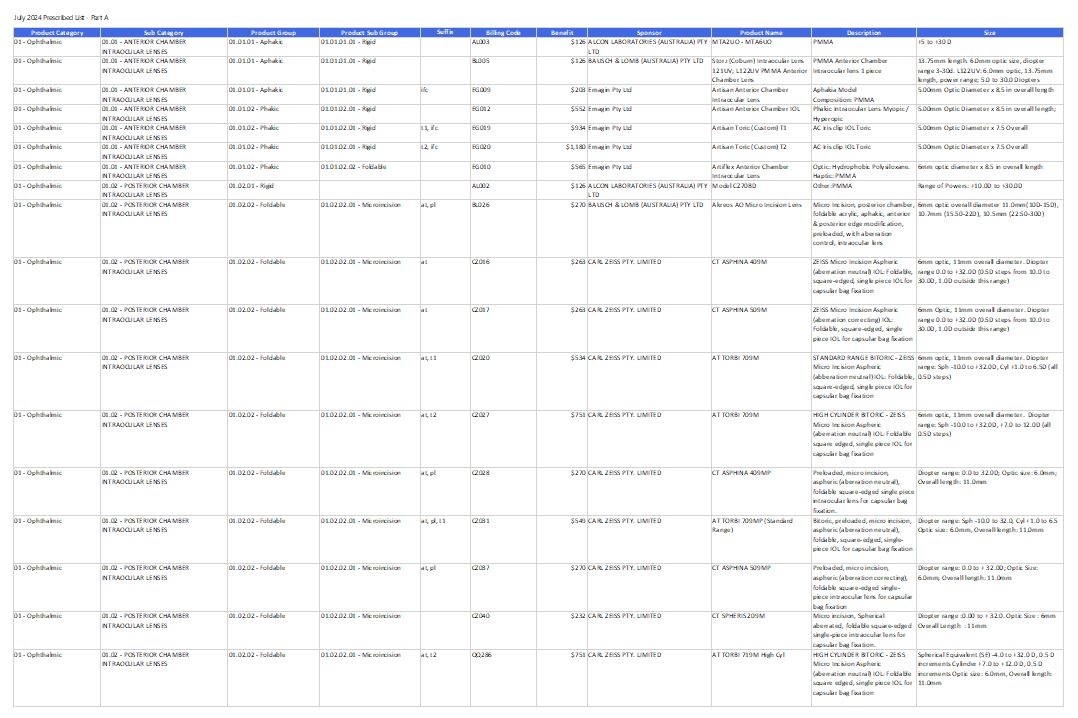
Prescribed List of Medical Devices and Human Tissue Products
This page lists the medical devices and human tissue products private health insurers must pay benefits for to the patients who have appropriate insurance policies. The latest version of the Prescribed List is effective from 1 July 2025. -

Stakeholder feedback summary – Consultation Paper 9 – CIED and the cost of technical support services
This document provides a high-level summary of stakeholder feedback received in response to Consultation Paper 9 – CIED and the cost of technical support services. -

Prescribed List post-listing review framework
We have developed a Prescribed List Post-listing Review Framework that is used to guide the post-listing review process as part of the Prescribed List Reforms. -

Interim Evaluation #1 of the Prescribed List Reforms
The interim evaluation report covers the period from May 2021 to June 2024 and reports on whether the reforms being implemented and achieving the expected outcomes. -

Reforms to the Prescribed List Part B – Analysis of stakeholder feedback
This report is to provide an analysis of stakeholder feedback received. -

Regional, Rural and Remote Home Care Workforce Support – Program Manual 2024-2025
This document provides information on the Regional, Rural and Remote Home Care Workforce Support Program, and is designed for grant applicants and recipients. -

Baseline evaluation of the Prostheses List reforms – Final report
This evaluation focuses on the Prostheses List reforms, which are running from 1 July 2021 to 30 June 2025. Evaluation of these reforms will determine whether the reform program is being implemented as intended. -
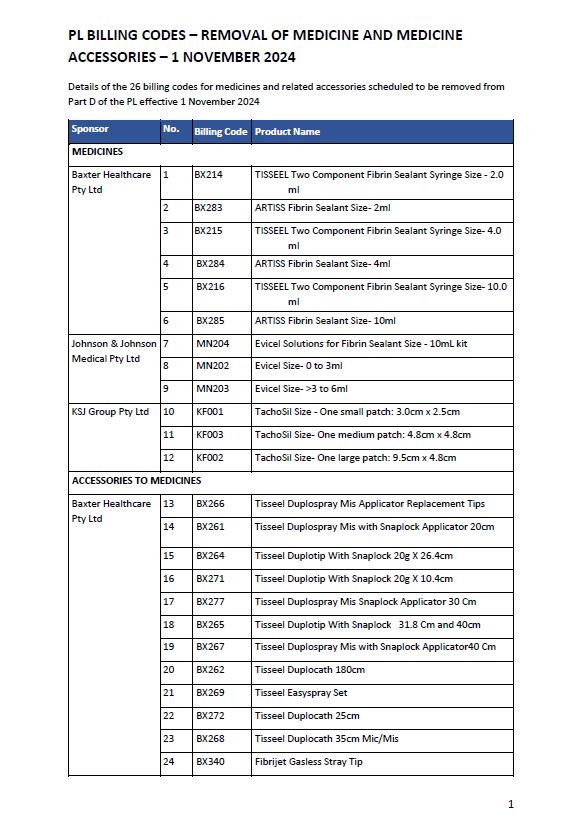
Prescribed List billing codes – Removal of medicine and medicine accessories – 1 November 2024
This list includes details of the 26 billing codes for medicines and related accessories scheduled to be removed from Part D of the Prescribed List effective 1 November 2024. -

Consultation Paper 7 – Proposed measures for compliance, assurance and information sharing
This report provides an analysis of stakeholder feedback received in response to the Consultation Paper on the proposed Compliance, Assurance and Information sharing measures for the Prescribed List (PL). -

Consultation Paper 8a – Gifts, benefits and discounts reporting requirement
This report provides an analysis of stakeholder feedback received in response to the consultation paper on the proposed measure: Prescribed List (PL) gifts, benefits and discounts reporting requirements. -

Consultation Paper 8b – Alignment of amount charged for supply of a device with corresponding PL benefit
This report provides an analysis of stakeholder feedback received in response to the consultation paper on the proposed measure: alignment of amount charged for supply of a device with corresponding PL benefit. -
 16:21
16:21Prescribed List – practical tips on submitting applications
This webinar recording provides practical tips on submitting applications. -

Prostheses List Post-Listing review – Consultants report
This report is a review of surgical guides and biomodels currently listed on the Prostheses List.
-
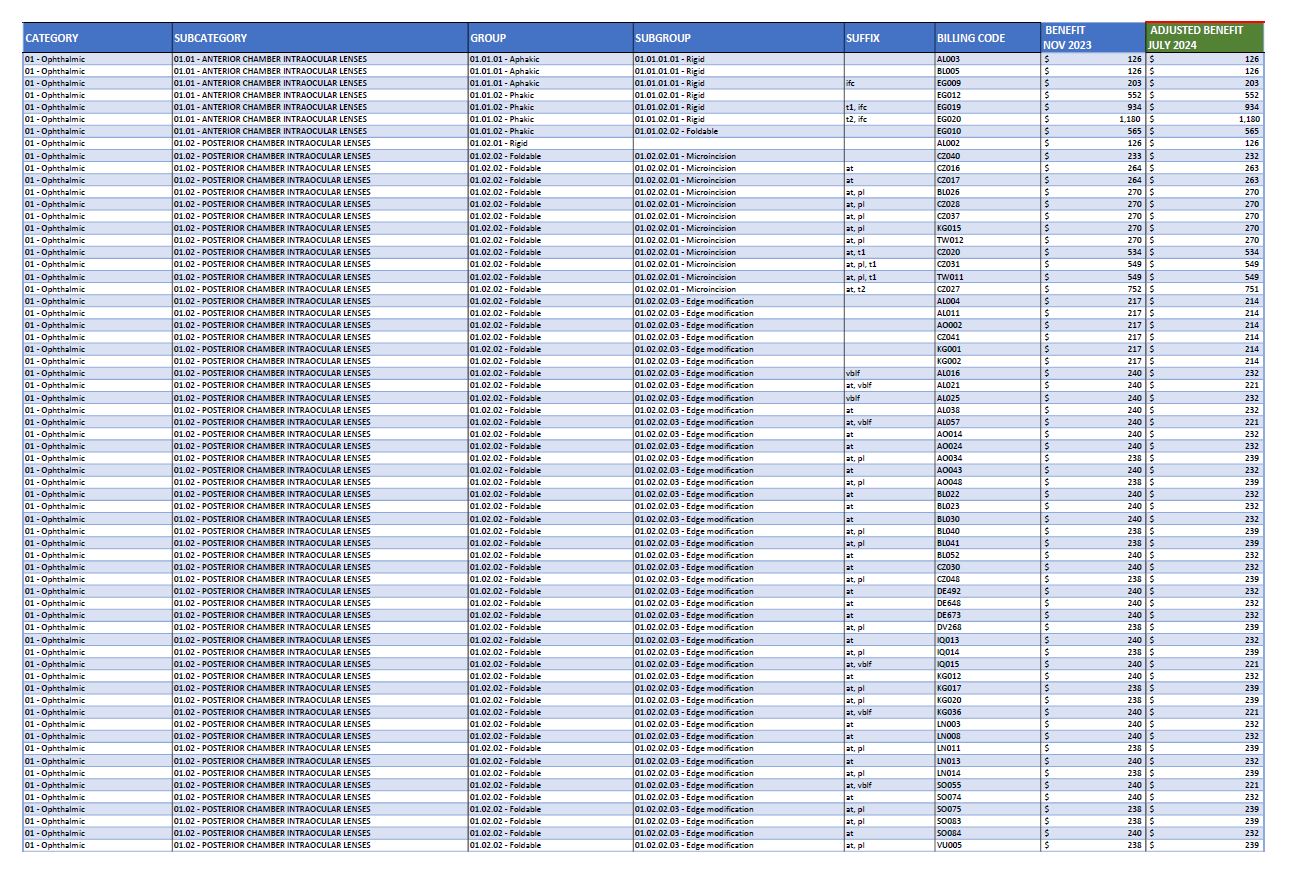
Advice on the Prescribed List adjusted benefit amounts
These documents contain advice on the Prescribed List (PL) adjusted benefits, resulting from the public benchmarking undertaken by the Independent Health and Aged Care Pricing Authority (IHACPA). The documents outline the November 2023 PL benefits, and the adjusted benefits effective 1 July 2024. -

Cost Recovery Implementation Statement – Administration of the Prescribed List of Benefits for Medical Devices and Human Tissue Products
This cost recovery implementation statement describes how the Department of Health and Aged Care recovers the costs of administering the Prescribed List of Benefits for Medical Devices and Human Tissue Products. -

Terms of reference – Medical Devices and Human Tissue Advisory Committee
Terms of reference for the Medical Devices and Human Tissue Advisory Committee. -
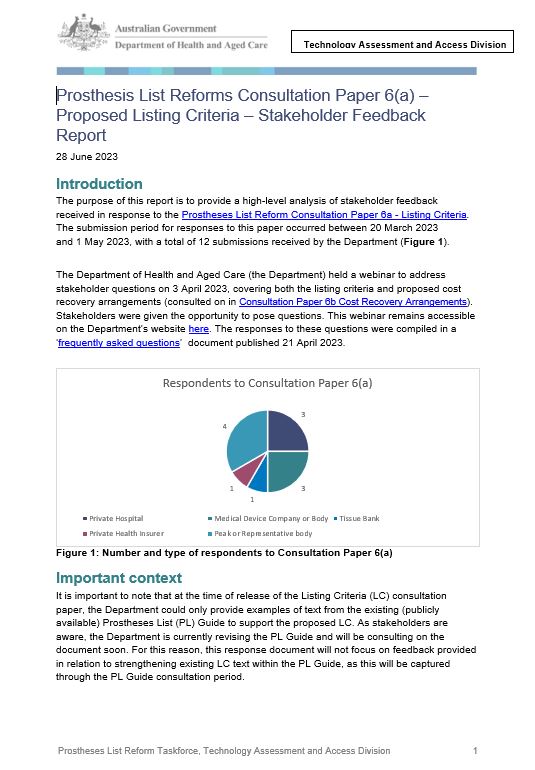
Stakeholder Feedback Report – Consultation Paper 6(a) Proposed Listing Criteria
This report provides an analysis of stakeholder feedback received in response to the Prostheses List Reform Consultation Paper 6a - Listing Criteria. -
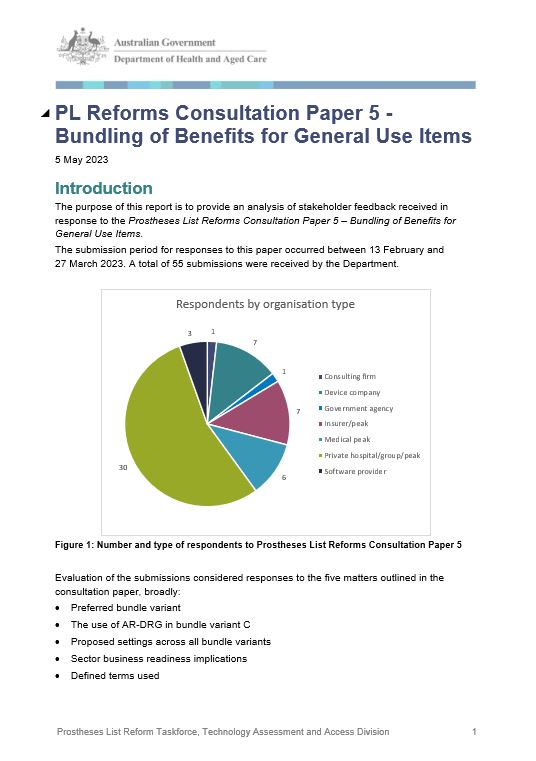
Stakeholder Feedback Report – Consultation Paper 5 Bundling of Benefits for General Use Items
This report provides an analysis of stakeholder feedback received in response to the Prostheses List Reform Consultation Paper 6a – Listing Criteria. -
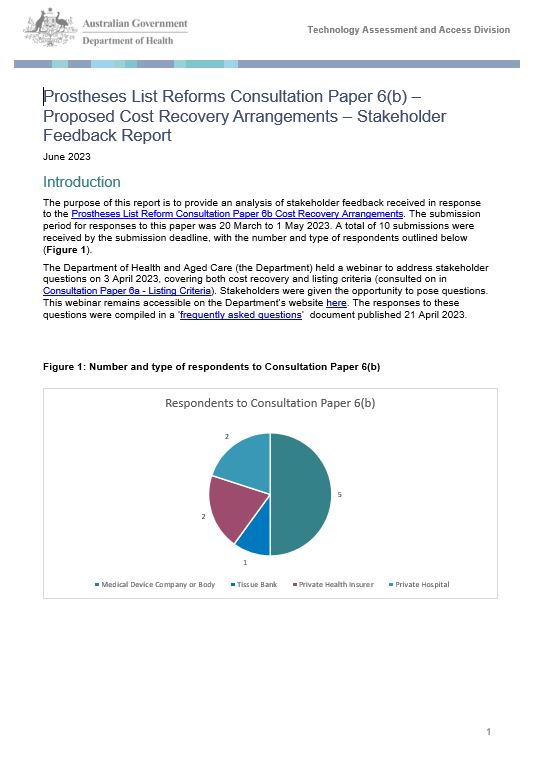
Stakeholder Feedback Report - Prostheses List Reforms Consultation Paper 6(b) Proposed Cost Recovery Arrangements
This report provides an analysis of stakeholder feedback received in response to the Prostheses List Reform Consultation Paper 6b Cost Recovery Arrangements. -

Prescribed List Compliance Strategy – Safeguarding the Prescribed List
The below document outlines Prescribed List compliance obligations for relevant stakeholders and the Australian Government. -
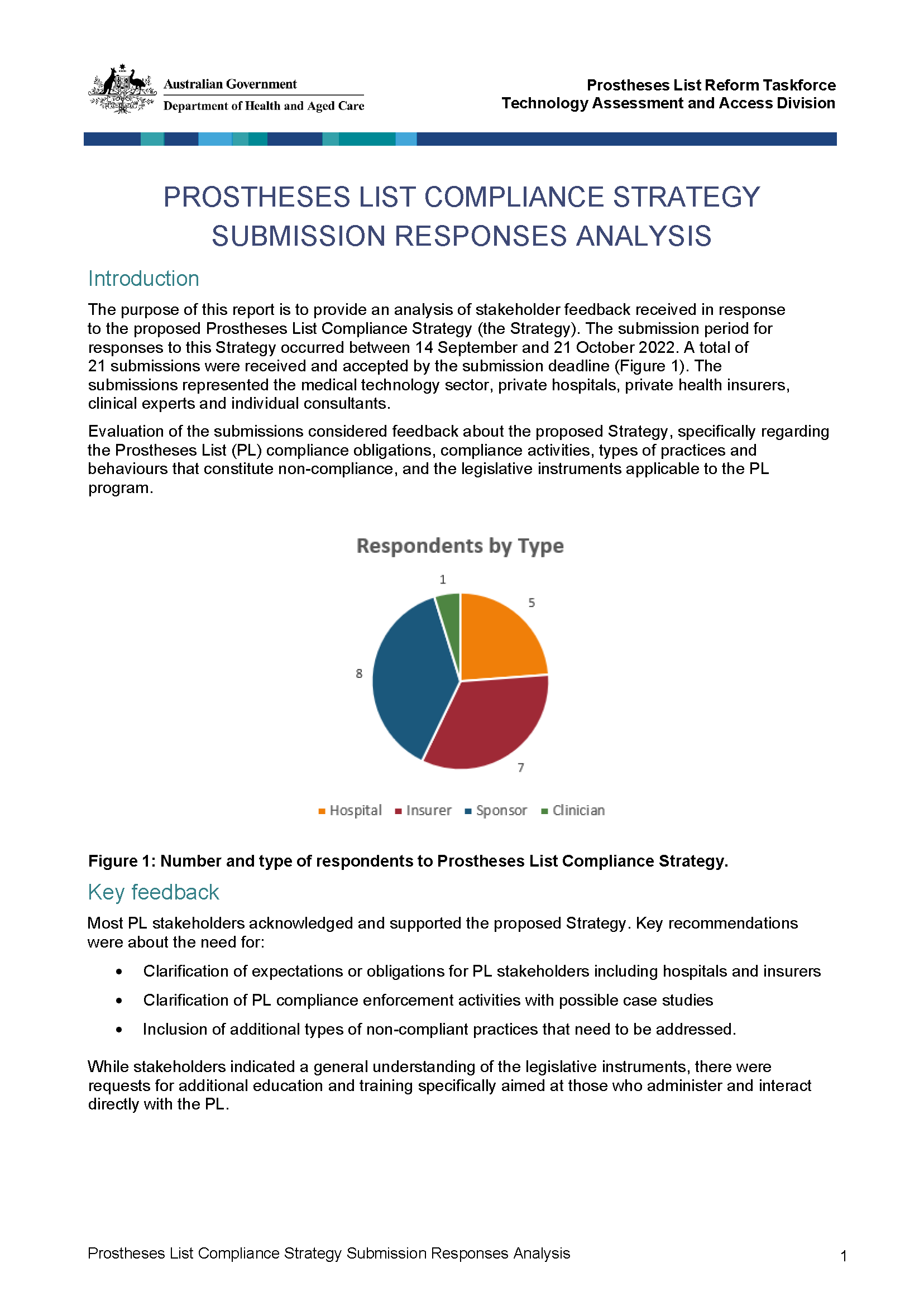
Submissions analysis on the Prostheses List Compliance Strategy
The below report provides analysis of stakeholder feedback to the proposed Prostheses List Compliance Strategy. The submission period for responses was between 14 September and 21 October 2022. -

Prostheses List cardiac implantable electronic devices (CIEDs) benefit reductions from 1 July 2023
The below data documents detail cardiac implantable electronic devices (CIEDs) that will have their Prostheses List (PL) benefits reduced by 40% of the gap on 1 July 2023. -

Supplementary advice on bundling arrangements for General Use Items on the Prostheses List
This report details supplementary advice on bundling arrangements for General Use Items on the Prostheses List.
