Filter results
You can narrow down the results using the filters
Audience
Topics
Our work
Year
111 results
-
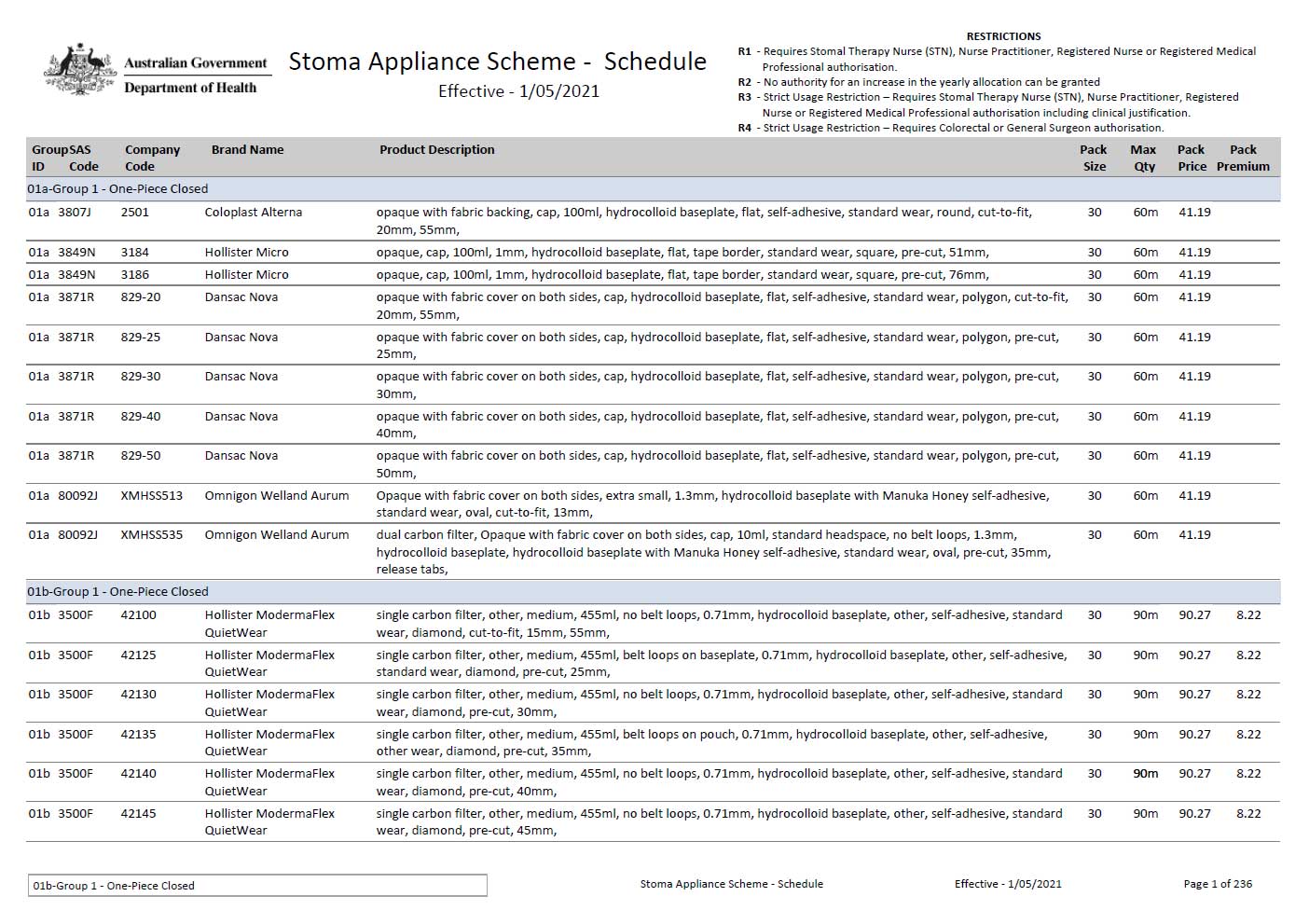
Stoma Appliance Scheme Schedule for download
The Stoma Appliance Scheme Schedule lists all the stoma-related products and appliances that we subsidise under the scheme. You can download the full list in PDF or Excel. -
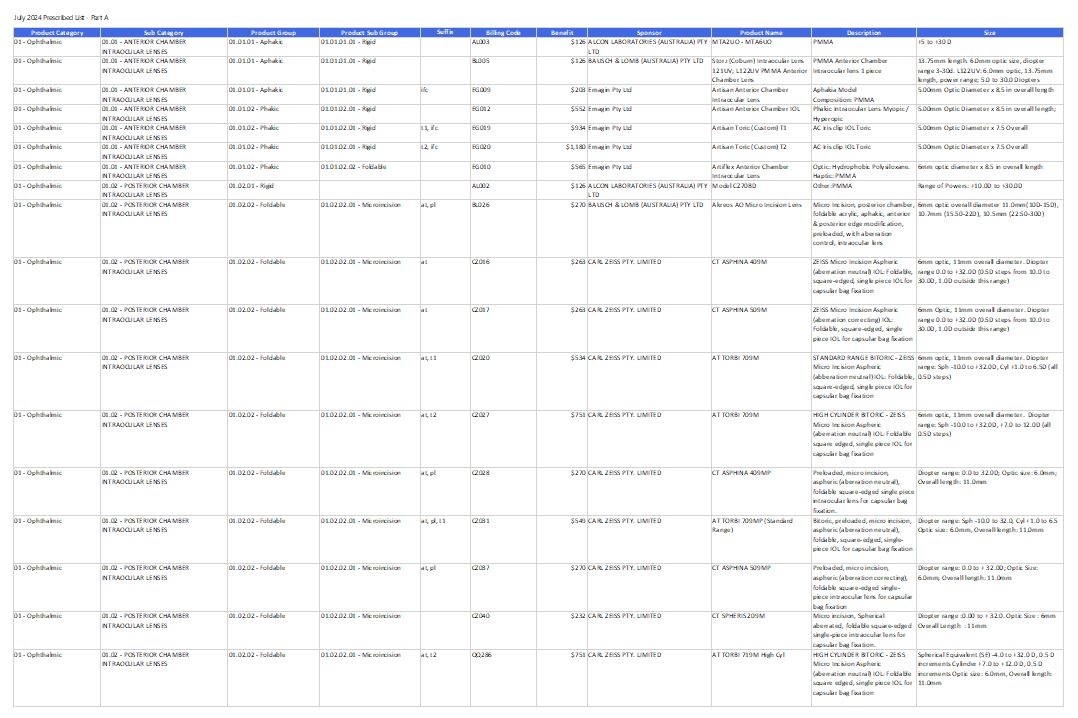
Prescribed List of Medical Devices and Human Tissue Products
This page lists the medical devices and human tissue products private health insurers must pay benefits for to the patients who have appropriate insurance policies. The latest version of the Prescribed List is effective from 1 March 2025. -
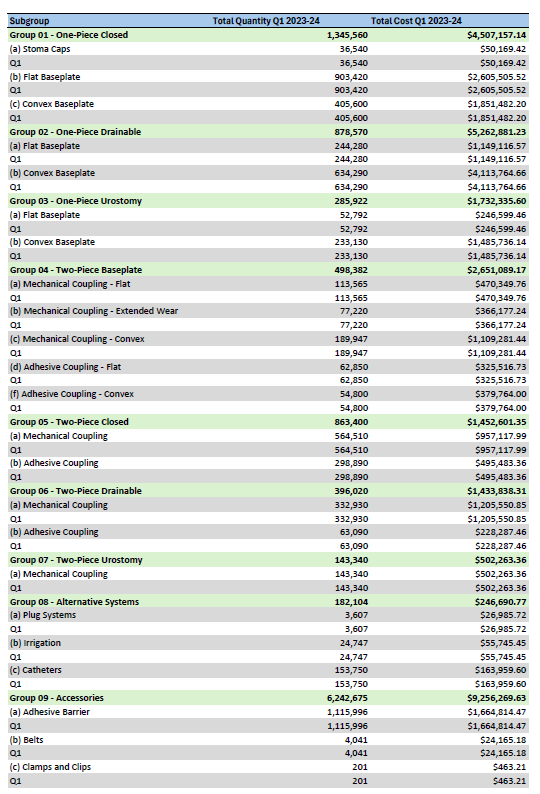
Stoma Appliance Scheme utilisation and expenditure data – Q1 – July to September 2023
This document shows how much we spent in July to September 2023 on stoma appliances and products under the Stoma Appliance Scheme in the 2023-2024 financial year. -
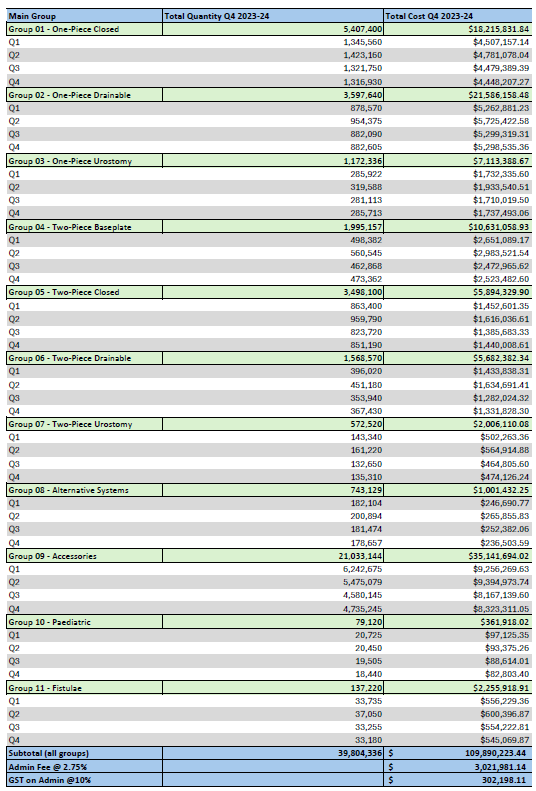
Stoma Appliance Scheme utilisation and expenditure data – Q2 – October to December 2023
This document shows how much we spent in October to December 2023 on stoma appliances and products under the Stoma Appliance Scheme in the 2023-2024 financial year. -
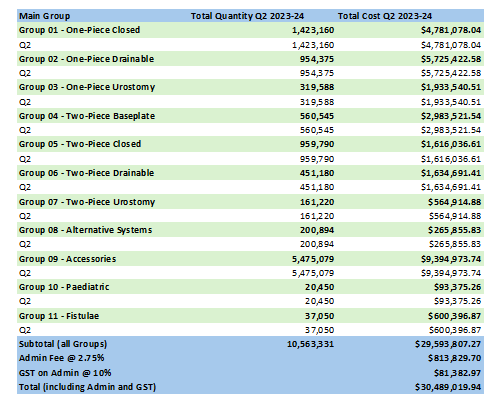
Stoma Appliance Scheme utilisation and expenditure data – Q4 – April to June and EOFY – Financial Year 2023–24
This document shows how much we spent in April to June 2024 and EOFY on stoma appliances and products under the Stoma Appliance Scheme in the 2023-2024 financial year. -
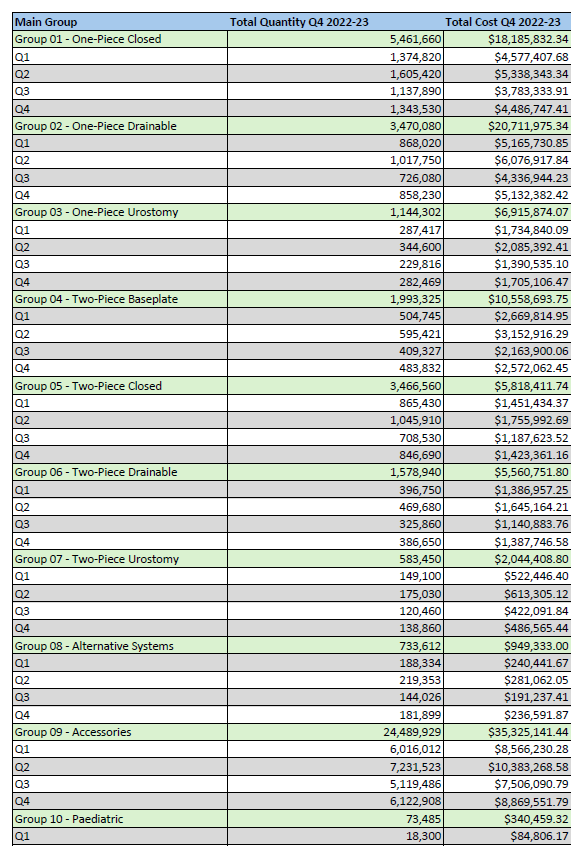
Stoma Appliance Scheme utilisation and expenditure data – Q4 – April to June and EOFY – Financial Year 2022–23
This document shows how much we spent in April to June 2023 and EOFY on stoma appliances and products under the Stoma Appliance Scheme in the 2022-2023 financial year. -
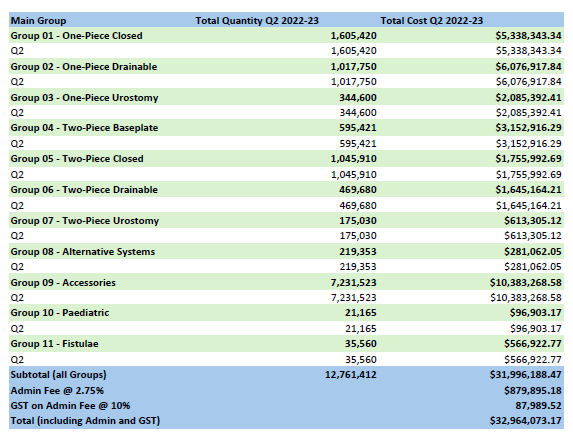
Stoma Appliance Scheme utilisation and expenditure data – Q2 – October to December 2022
This document shows how much we spent in October to December 2022 on stoma appliances and products under the Stoma Appliance Scheme in the 2022–2023 financial year. -
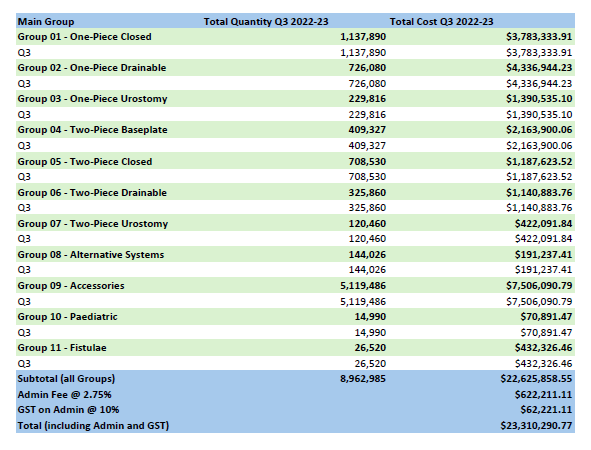
Stoma Appliance Scheme utilisation and expenditure data – Q3 – January to March 2023
This document shows how much we spent in January to March 2023 on stoma appliances and products under the Stoma Appliance Scheme in the 2022–2023 financial year. -
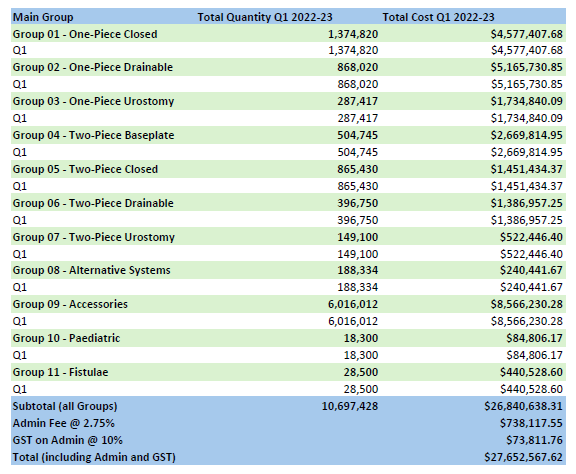
Stoma Appliance Scheme utilisation and expenditure data – Q1 – July to September 2022
This document shows how much we spent in July to September 2023 on stoma appliances and products under the Stoma Appliance Scheme in the 2022-2023 financial year. -

Stakeholder feedback summary – Consultation Paper 9 – CIED and the cost of technical support services
This document provides a high-level summary of stakeholder feedback received in response to Consultation Paper 9 – CIED and the cost of technical support services. -
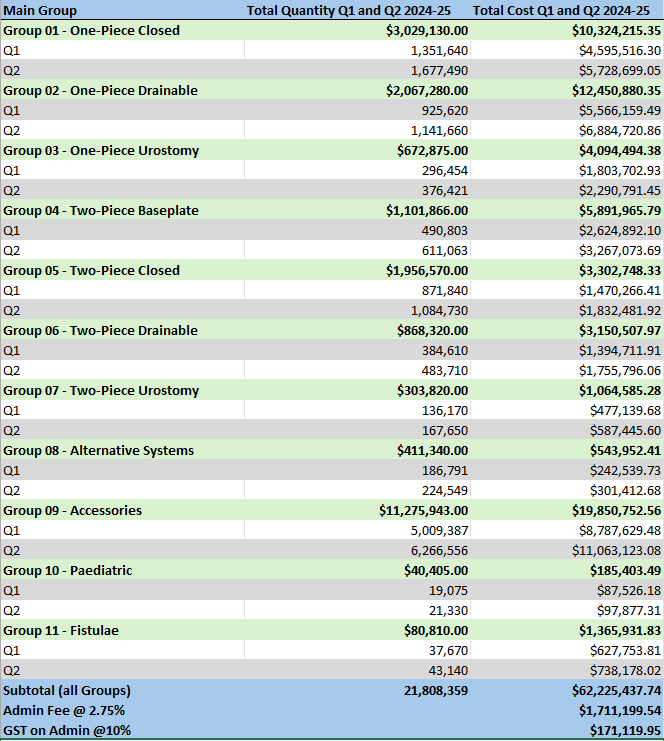
Stoma Appliance Scheme utilisation and expenditure data quarterly report – October to December 2024
This document shows how much we spent between October and November 2024 on stoma appliances and products under the Stoma Appliance Scheme. -

Interim Evaluation #1 of the Prescribed List Reforms
The interim evaluation report covers the period from May 2021 to June 2024 and reports on whether the reforms being implemented and achieving the expected outcomes. -
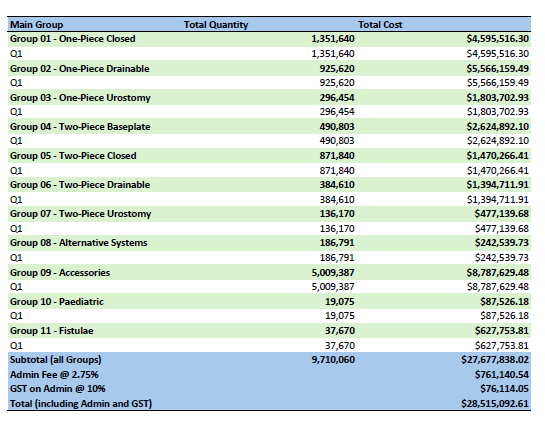
Stoma Appliance Scheme utilisation and expenditure data – July to September 2024
This document shows how much we spent between July to September 2024 on stoma appliances and products under the Stoma Appliance Scheme. -
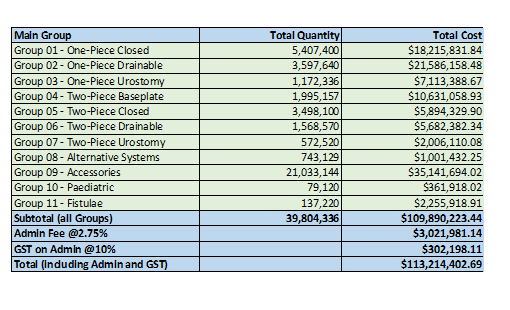
Stoma Appliance Scheme utilisation and expenditure data 2023-24
This document shows how much we spent in 2023-24 on stoma appliances and products under the Stoma Appliance Scheme. -
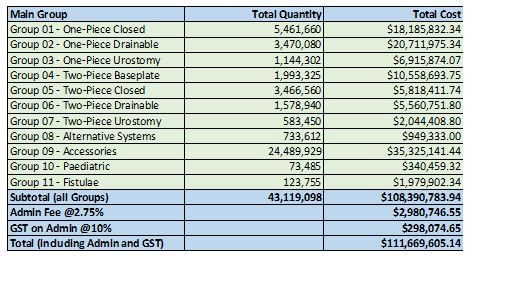
Stoma Appliance Scheme utilisation and expenditure data 2022-23
This document shows how much we spent in 2022-23 on stoma appliances and products under the Stoma Appliance Scheme. -

Consultation Paper 7 – Proposed measures for compliance, assurance and information sharing
This report provides an analysis of stakeholder feedback received in response to the Consultation Paper on the proposed Compliance, Assurance and Information sharing measures for the Prescribed List (PL). -

Consultation Paper 8a – Gifts, benefits and discounts reporting requirement
This report provides an analysis of stakeholder feedback received in response to the consultation paper on the proposed measure: Prescribed List (PL) gifts, benefits and discounts reporting requirements. -

Consultation Paper 8b – Alignment of amount charged for supply of a device with corresponding PL benefit
This report provides an analysis of stakeholder feedback received in response to the consultation paper on the proposed measure: alignment of amount charged for supply of a device with corresponding PL benefit. -
 16:21
16:21Prescribed List – practical tips on submitting applications
This webinar recording provides practical tips on submitting applications. -

National Clinical Quality Registry Program – Communique, August 2024
This communique provides an update on the National Clinical Quality Registry Program in August 2024. It covers upcoming funding opportunities and other news and events. -
National Clinical Quality Registry Program – Communiques
This collection contains communiques that provide updates on the National Clinical Quality Registry Program. -

Prostheses List Post-Listing review – Consultants report
This report is a review of surgical guides and biomodels currently listed on the Prostheses List.
-

Urogynaecological mesh devices (mid-urethral slings)
This report summarises the process, findings, and outcome of our post-listing review of urogynaecological mesh devices. -

Metal-backed patellae
This report summarises the process, findings, and outcome of our post-listing review of metal-backed patellae. -

Spinal cord stimulators
This report was prepared by Health Research Consulting and summarises their review into clinical and cost effectiveness of spinal cord stimulators. The information in this report was considered during our post-listing review of spinal cord stimulators.

