Filter results
You can narrow down the results using the filters
Audience
Topics
Our work
Year
93 results
-
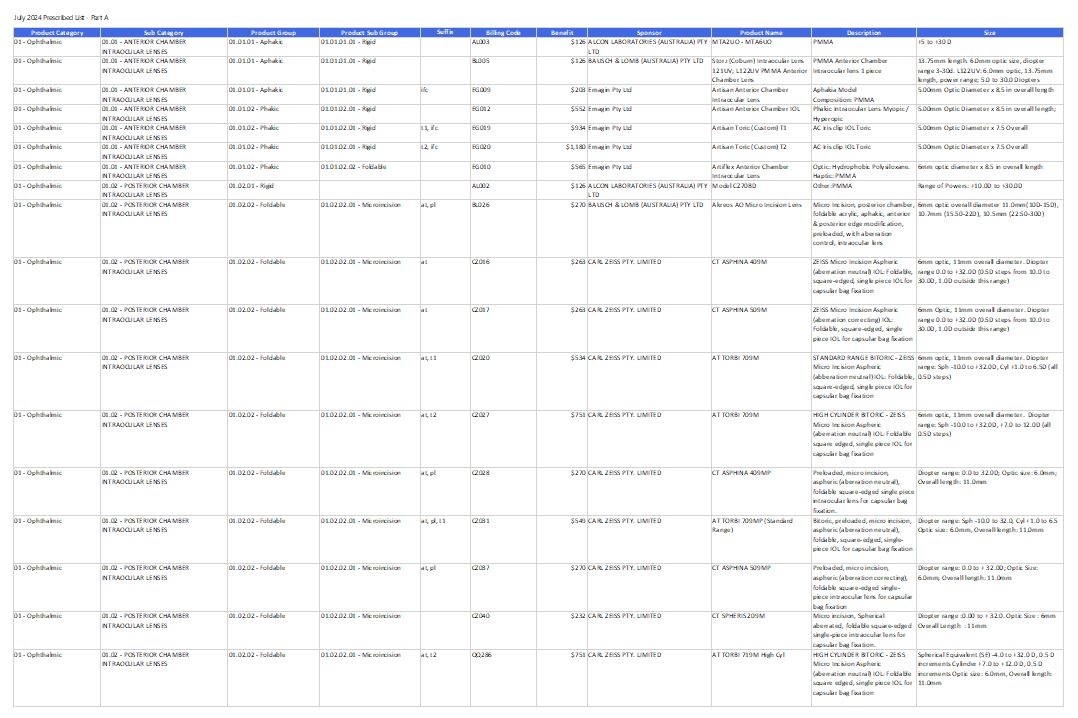
Prescribed List of Medical Devices and Human Tissue Products
This page lists the medical devices and human tissue products private health insurers must pay benefits for to the patients who have appropriate insurance policies. The latest version of the Prescribed List is effective from 1 March 2025. -

Stakeholder feedback summary – Consultation Paper 9 – CIED and the cost of technical support services
This document provides a high-level summary of stakeholder feedback received in response to Consultation Paper 9 – CIED and the cost of technical support services. -

Prescribed List post-listing review framework
We have developed a Prescribed List Post-listing Review Framework that is used to guide the post-listing review process as part of the Prescribed List Reforms. -

BreastScreen Australia Data Dictionary
This data dictionary contains definitions used by the BreastScreen Australia Program so that data collected for monitoring, evaluating and accrediting services is consistent across Australia. -

Consultation Paper 7 – Proposed measures for compliance, assurance and information sharing
This report provides an analysis of stakeholder feedback received in response to the Consultation Paper on the proposed Compliance, Assurance and Information sharing measures for the Prescribed List (PL). -

Consultation Paper 8a – Gifts, benefits and discounts reporting requirement
This report provides an analysis of stakeholder feedback received in response to the consultation paper on the proposed measure: Prescribed List (PL) gifts, benefits and discounts reporting requirements. -

Consultation Paper 8b – Alignment of amount charged for supply of a device with corresponding PL benefit
This report provides an analysis of stakeholder feedback received in response to the consultation paper on the proposed measure: alignment of amount charged for supply of a device with corresponding PL benefit. -
 16:21
16:21Prescribed List – practical tips on submitting applications
This webinar recording provides practical tips on submitting applications. -

Prostheses List Post-Listing review – Consultants report
This report is a review of surgical guides and biomodels currently listed on the Prostheses List.
-
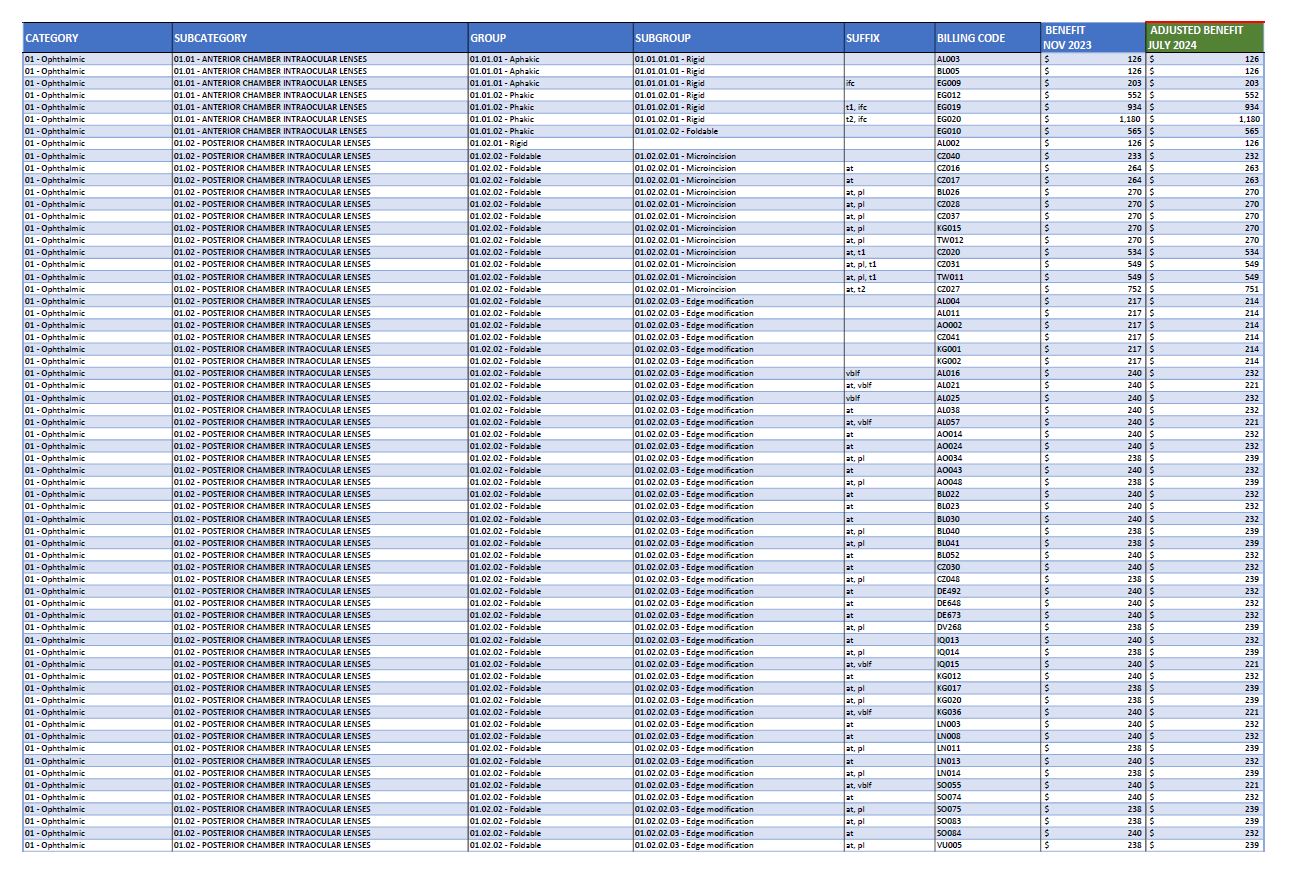
Advice on the Prescribed List adjusted benefit amounts
These documents contain advice on the Prescribed List (PL) adjusted benefits, resulting from the public benchmarking undertaken by the Independent Health and Aged Care Pricing Authority (IHACPA). The documents outline the November 2023 PL benefits, and the adjusted benefits effective 1 July 2024. -

Cost Recovery Implementation Statement – Administration of the Prescribed List of Benefits for Medical Devices and Human Tissue Products
This cost recovery implementation statement describes how the Department of Health and Aged Care recovers the costs of administering the Prescribed List of Benefits for Medical Devices and Human Tissue Products. -

Terms of reference – Medical Devices and Human Tissue Advisory Committee
Terms of reference for the Medical Devices and Human Tissue Advisory Committee. -

Prescribed List Compliance Strategy – Safeguarding the Prescribed List
The below document outlines Prescribed List compliance obligations for relevant stakeholders and the Australian Government. -
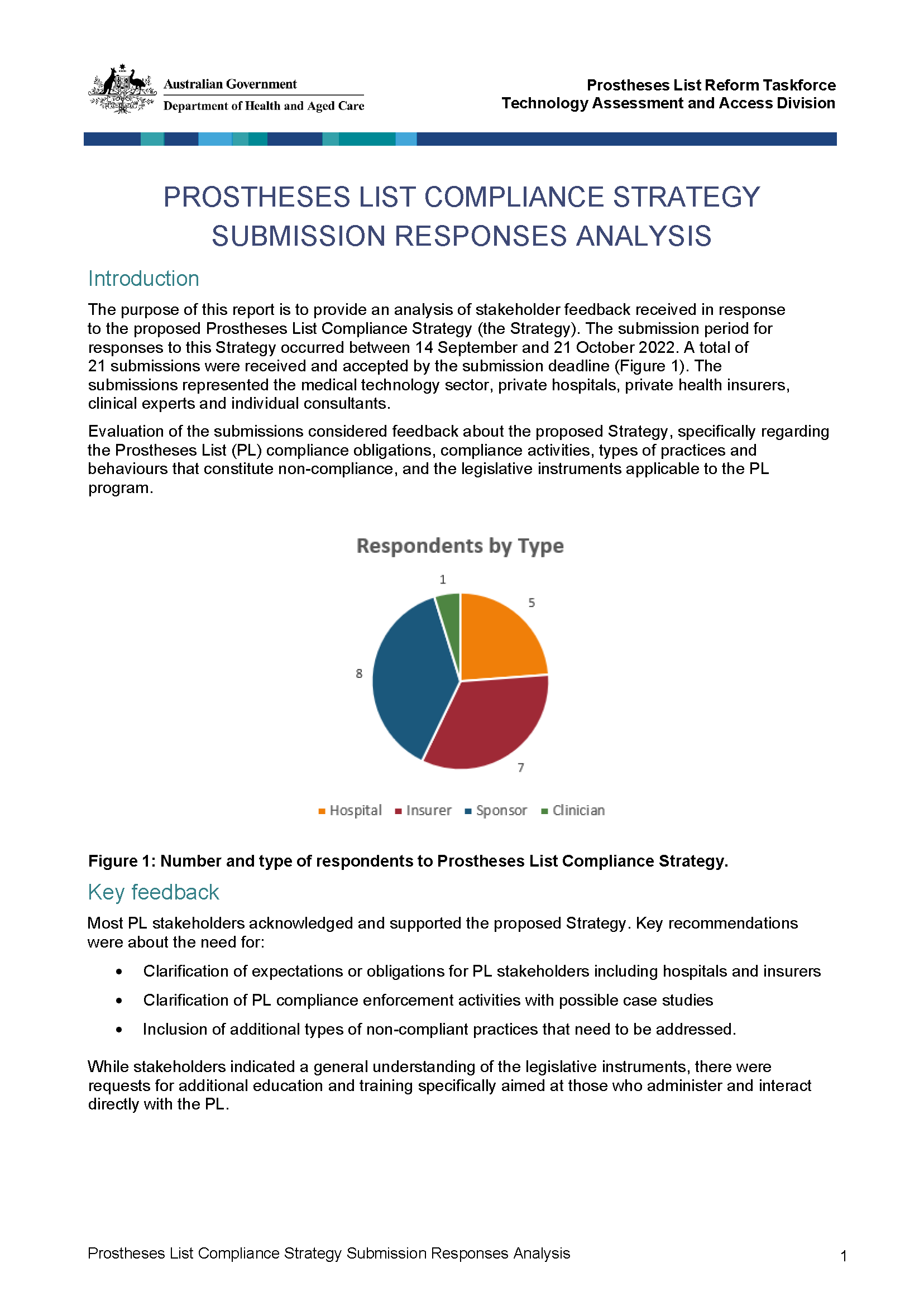
Submissions analysis on the Prostheses List Compliance Strategy
The below report provides analysis of stakeholder feedback to the proposed Prostheses List Compliance Strategy. The submission period for responses was between 14 September and 21 October 2022. -

Prostheses List cardiac implantable electronic devices (CIEDs) benefit reductions from 1 July 2023
The below data documents detail cardiac implantable electronic devices (CIEDs) that will have their Prostheses List (PL) benefits reduced by 40% of the gap on 1 July 2023. -
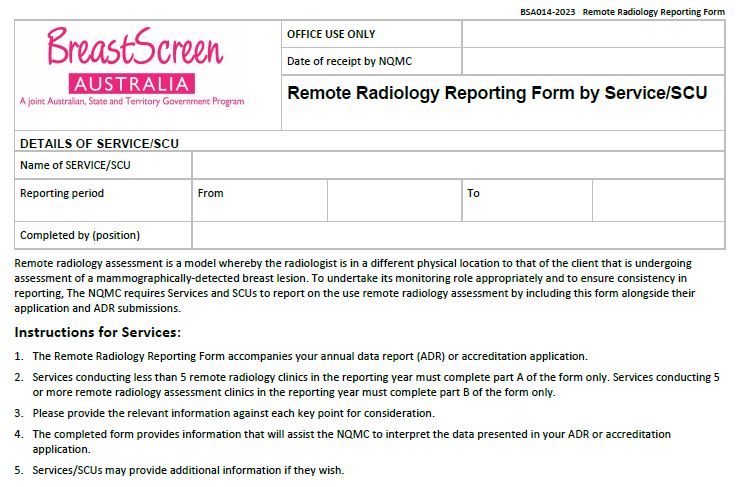
BreastScreen Australia – Remote radiology reporting form
BreastScreen Australia services and State Coordination Units (SCUs) use this form for Remote radiology reporting by Service/SCU. -

Prostheses List Reforms – Pre-listing Assessment framework and Governance structure
This report outlines the pre-listing assessment framework and governance structure for the Prostheses List. It also addresses Ernst & Young, and Adelaide Health Technology Assessment options and recommendations. -

Options for a reformed Prostheses List pre-listing assessment framework and governance structure
This report provides a framework and proposes options for governance that would align the Prostheses List processes with those of other health technology assessment processes within the Government. It was developed by Adelaide Health Technology Assessment, under the Prostheses List Reform program. -

Review of the Prostheses List advisory committee and associated subcommittees
This review provides options and recommendations for the future governance of the Prostheses List. It was developed by Ernst & Young under the Department’s Prostheses List Reform program. -

Advice on Bundling Arrangements for General Use Items on the Prostheses List
A report from the Independent Health and Aged Care Pricing Authority (IHACPA) detailing advice on the bundling arrangements for General Use Items currently listed on the Prostheses List (PL). -

Cost Recovery Implementation Statement – Administration of the Prostheses List – 1 July 2022 to 30 June 2023
This statement describes how the Department of Health recovers the costs of administering the Prostheses List. -
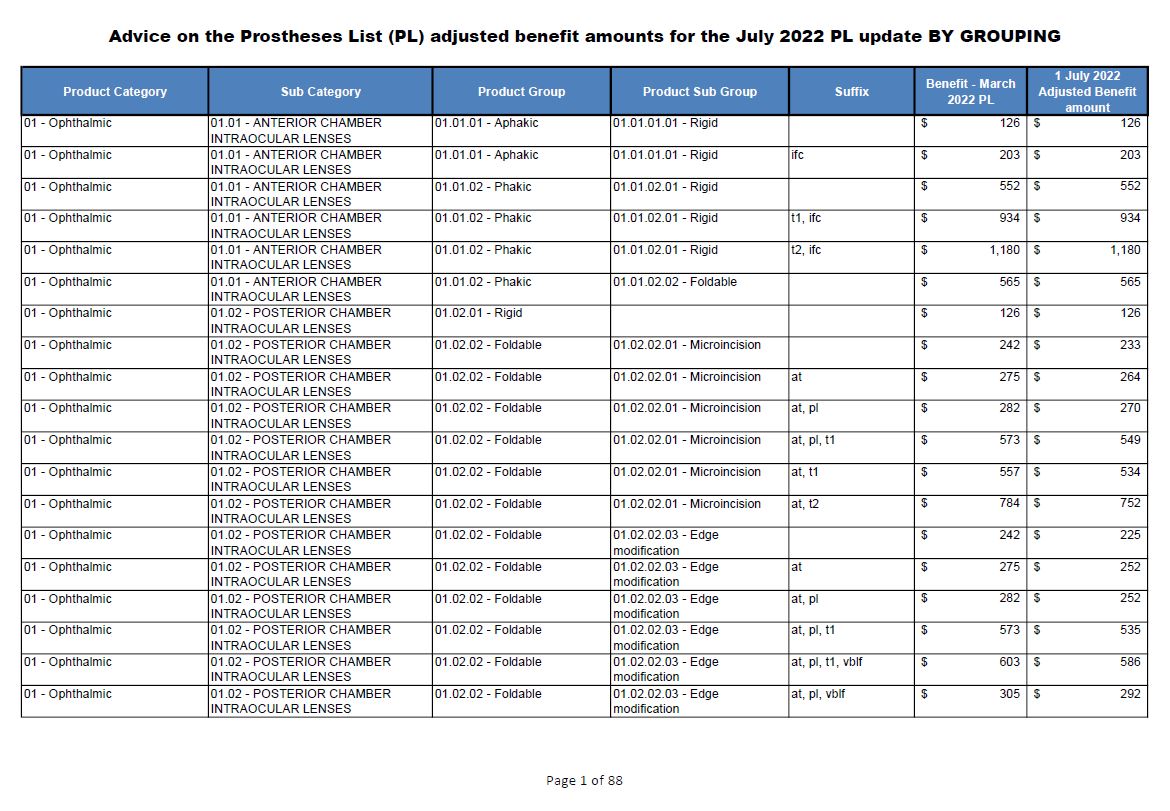
Advice on the Prostheses List adjusted benefit amounts
This collection of document contains the advice on the Prostheses List adjusted benefit amounts resulting from the public benchmarking undertaken by the Independent Hospital Pricing Authority (IHPA). Each document shows the March 2022 benefits and the new benefits from 1 July 2022. -

Benchmark Price for Prostheses in Australian Public Hospitals 2020–21
This report was prepared by the Independent Hospital Pricing Authority (IHPA) to establish a benchmark price for prostheses in Australian public hospitals. -
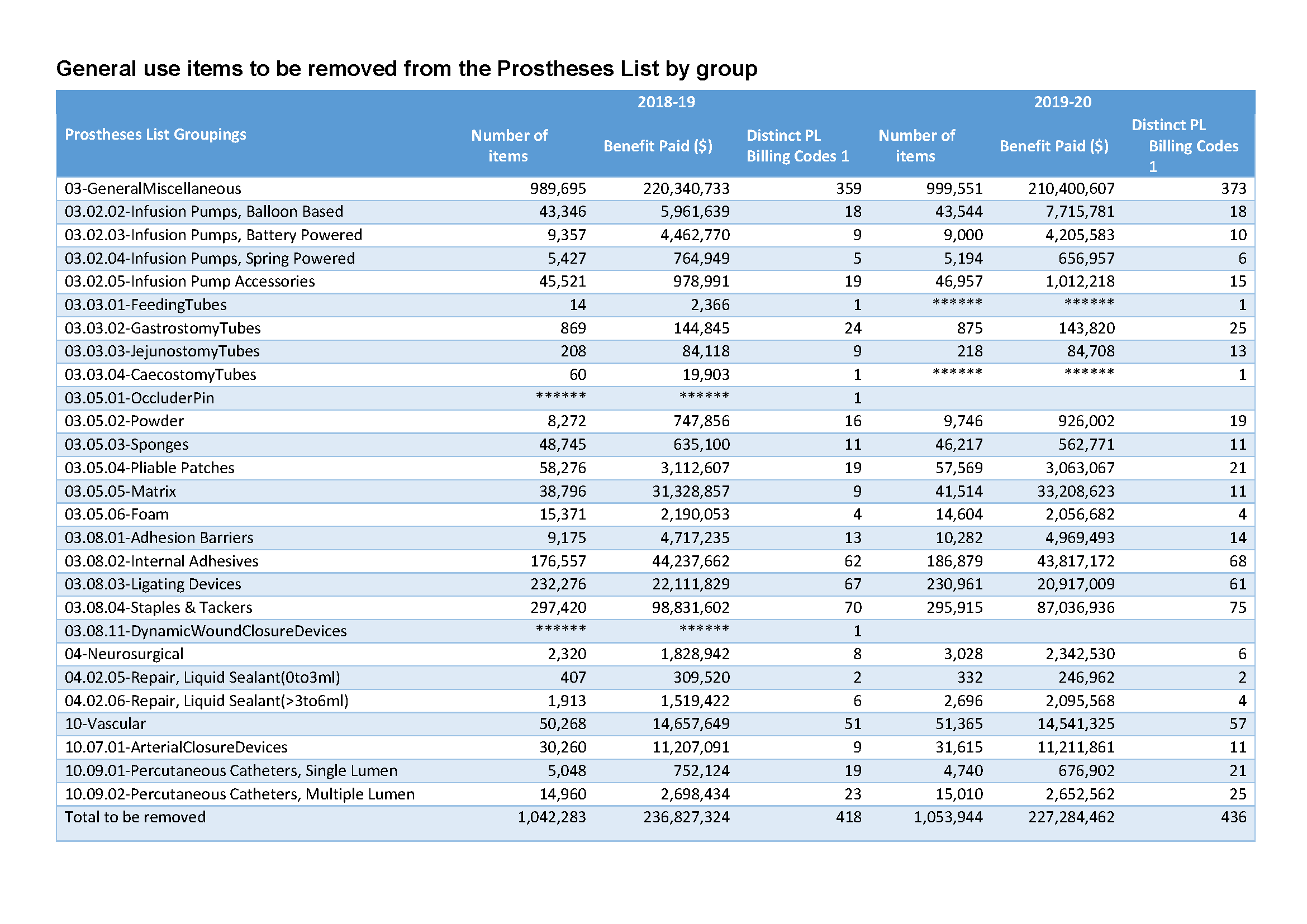
General use items to be removed from the Prostheses List by group
Updated final list of general use items from part A to be removed from the Prostheses List which includes data from the published March 2022 PL. -

Hospital Establishment – Type by Prostheses list product group
A list of all the items in the Prostheses list by private hospital type.
