Filter results
You can narrow down the results using the filters
Audience
Publication type
Topics
Our work
Year
26 results
-
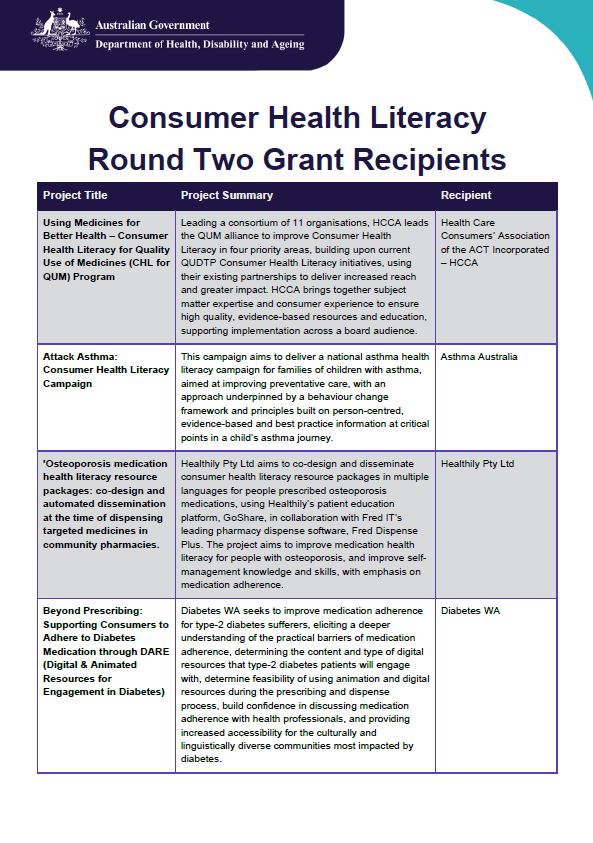
Consumer Health Literacy and Health Professional Education Grant Recipients
Grant opportunities for Consumer Health Literacy and Health Professional Education projects were sought in 2024. The Department received more than 50 applications and these were considered by an Assessment Committee. Here are some of the successful organisations and their projects. -

Handbook of tools to support medicine management in multimorbidity and polypharmacy
We commissioned the University of South Australia to develop this handbook which is designed to support medicine management in multimorbidity and polypharmacy. -

Presentation slides – Consumer Health Literacy and Health Professional Education Grant Opportunity Forum – QUDTP Program
Learn about the upcoming grant opportunities in the Quality Use of Diagnostics, Therapeutics and Pathology (QUDTP) Program. -

National PDS Fact Sheet for Clinicians and Pharmacists
This document includes information to help clinicians and pharmacists connect to the National Prescription Delivery Service. -

Connecting to the National Prescription Delivery Service
This document includes information to help developers connect to the National Prescription Delivery Service. -

Quality Use of Pathology Program (QUPP) project grants 2021–23
This document provides feedback to applicants for the Quality Use of Pathology Program Projects grant round (2021–23) GO5049 that was open on GrantConnect from 3 September 2021 to 19 October 2021. -

Care for your health – Your guide to electronic prescribing
This fact sheet outlines accessing medicines through electronic prescriptions. -
Quality Use of Pathology Program (QUPP) – Final report for the Common Sense Pathology 2018–2021 project
This final report outlines what The Royal College of Pathologists of Australasia Common Sense Pathology project achieved. The QUPP funded this project between 2018 and 2021. -

Electronic Prescribing Active Script List Privacy Framework
The Electronic Prescribing Active Script List (ASL) Privacy Framework describes the privacy, identity and consent processes that relate to release 1 of the implementation of Active Script List functionality. -
Quality Use of Pathology Program (QUPP) – Final report for the Pathology, Information, Terminology and Units Standardisation project (PITUS 18–20)
This final report outlines what The Royal College of Pathologists of Australasia (RCPA) PITUS 18–20 project achieved. The QUPP funded previous phases of this project. -

Electronic Prescriptions Privacy Policy
This policy outlines the privacy responsibilities of non-government organisations involved in the electronic prescribing process. -

Electronic Prescriptions Data Usage Policy
This policy describes how personal information and related data contained in electronic prescriptions is collected, used and disclosed. -

Summary of Privacy Impact Assessment on the Electronic Prescribing Project
This report details the Summary of Privacy Impact Assessment on the Electronic Prescribing Project. Electronic Prescribing is being implemented to increase PBS compliance and efficiency, improve drug safety, and support better data collection. -

Electronic Prescriptions Security and Access Policy
The Electronic Prescriptions Security and Access Policy describes how health care provider organisations must manage the security and integrity of their software, to make sure they dispense electronic prescriptions securely. -

Quality Use of Pathology Program (QUPP) – Final report for the structured pathology reporting of cancer in Australia 2017–20 project
This final report outlines the results of the Royal College of Pathologists of Australasia structured pathology reporting of cancer in Australia 2017–20 project. -

Quality Use of Pathology Program (QUPP) – Final report for the development of quality assurance programs for DNA extraction project
This final report outlines the results of the Royal College of Pathologists of Australasia Quality Assurance Programs project to develop quality assurance programs for DNA extraction. -

Quality Use of Pathology Program (QUPP) – Final report for the enhancement of the Lab Tests Online website project
This final report outlines what the Australasian Association of Clinical Biochemistry and Laboratory Medicine enhancement of the Lab Tests Online website project achieved. -

Quality Use of Pathology Program (QUPP) – Final report for the development and pilot of a quality assurance program
This final report outlines the results of the Royal College of Pathologists of Australasia Quality Assurance Programs pilot project for a quality assurance program for proficiency testing in cardiovascular disease. -

Quality Use of Pathology Program (QUPP) – Final report for the exploration of integrated NATA–RCPAQAP predictive modelling
This final report outlines what the Australian National University exploration of predictive integrated National Association of Testing Authorities (NATA) and Royal College of Pathologists of Australasia Quality Assurance Programs (RCPAQAP) modelling to improve pathology quality project achieved.
-

Quality Use of Pathology Program (QUPP) – Final report for the enhancing patient outcomes
This final report outlines the results of the Macquarie University project to enhance patient outcomes through evaluation of the appropriateness and quality use of pathology in general practice. -

Quality Use of Pathology Program (QUPP) – Final report for the Genetic and genomic certification for non-genetic pathologists and trainees
This final report outlines what The Royal College of Pathologists of Australasia (RCPA) genetic and genomic certification project achieved. -

Form of the Electronic Prescription 2019
The Form of the Electronic Prescription 2019 defines the information fields required when a PBS prescriber writes an electronic prescription. -

Electronic Prescriptions Information Technology Requirements Instrument 2019
The Electronic Prescriptions Information Technology Requirements Instrument 2019 details system requirements for participating in electronic prescribing and refers to system obligations to adhere to security, privacy and data usage policies. -

Quality Use of Pathology Program (QUPP) – Final report for the development of a mass spectrometry technical quality assurance program
This final report outlines the results of the Royal College of Pathologists of Australasia Quality Assurance Programs project to develop a development of a mass spectrometry technical quality assurance program for detecting human disease-associated proteins (Proteomics). -
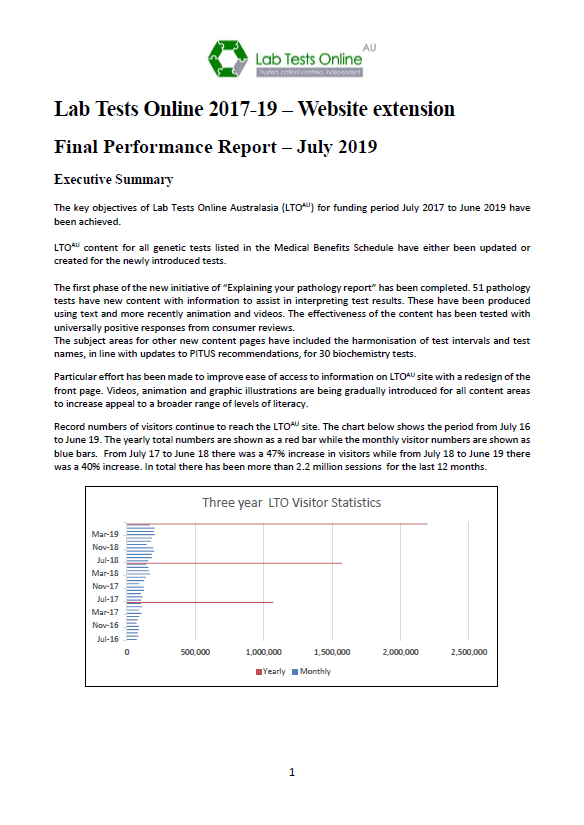
Quality Use of Pathology Program (QUPP) – Final report for the Lab Tests Online Australasia additions to support genetics, eHealth and outreach
This final report outlines the results of the Australasian Association of Clinical Biochemistry and Laboratory Medicine’s project to support genetics, eHealth and outreach.
