Filter results
You can narrow down the results using the filters
Audience
Topics
Our work
Year
129 results
-

Life Saving Drugs Program (LSDP) Expert Panel meeting agenda – 25 July 2025
Life Saving Drugs Program (LSDP) Expert Panel (the panel) agenda for the 17th meeting on 25 July 2025. -
Incentives and support for GPs and general practices in MM locations
This collection contains the Modified Monash (MM) categories MM 1 to MM 7 fact sheets. -

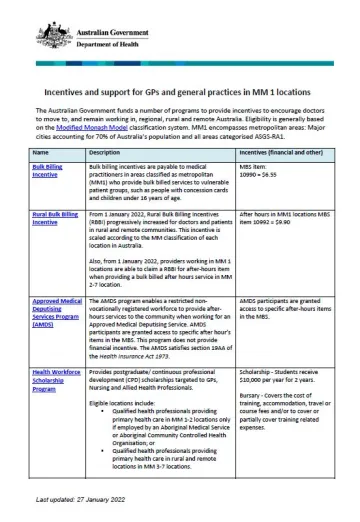
Incentives and support for GPs and general practices in Modified Monash 1 locations
The Modified Monash (MM) fact sheet for category MM 1 covers metropolitan areas. -

Incentives and support for GPs and general practices in Modified Monash 2 locations
The Modified Monash (MM) fact sheet for category MM 2 covers regional areas. -
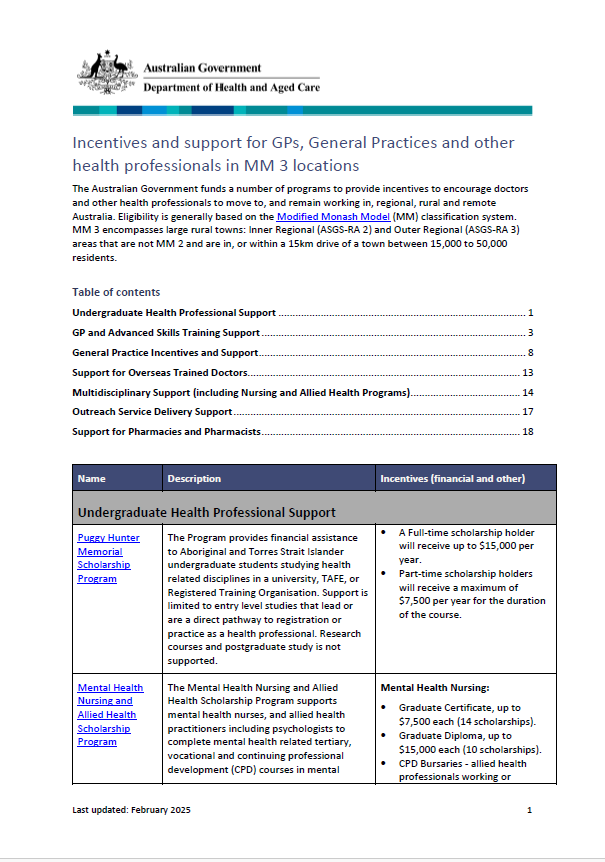
Incentives and support for GPs and general practices in Modified Monash 3 locations
The Modified Monash (MM) fact sheet for category MM 3 covers rural towns. -
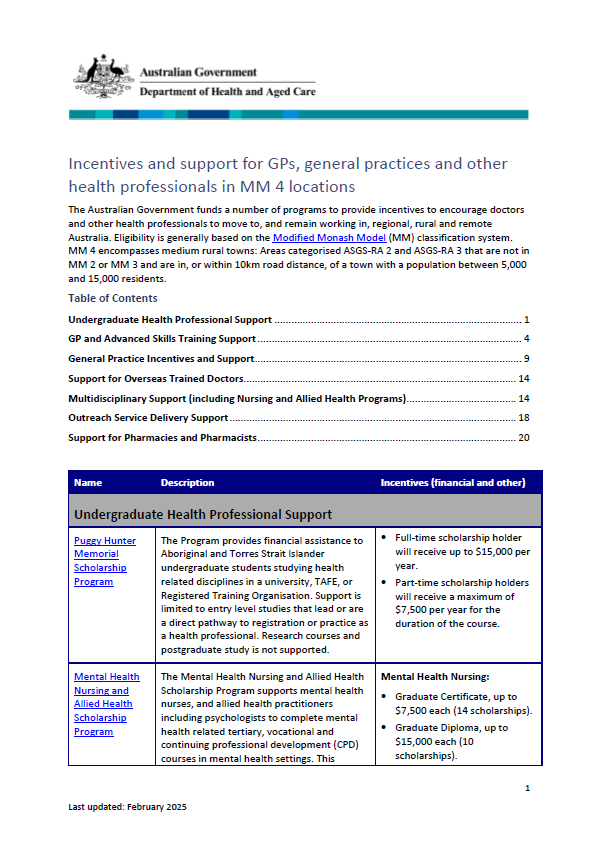
Incentives and support for GPs and general practices in Modified Monash 4 locations
The Modified Monash (MM) fact sheet for category MM 4 covers medium rural towns. -

Incentives and support for GPs and general practices in Modified Monash 5 locations
The Modified Monash (MM) fact sheet for category MM 5 covers small rural towns. -

Incentives and support for GPs and general practices in Modified Monash 6 locations
The Modified Monash (MM) fact sheet for category MM 6 covers remote communities. -
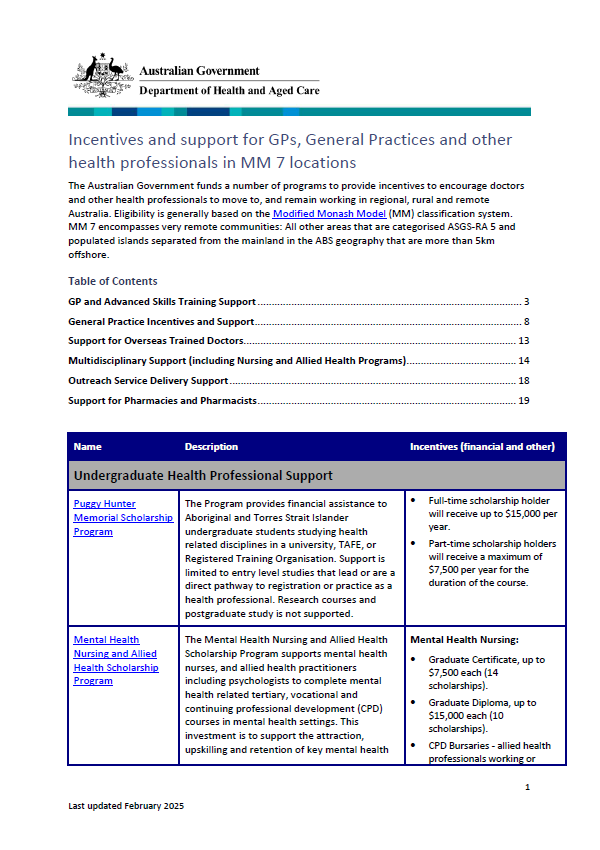
Incentives and support for GPs and general practices in Modified Monash 7 locations
The Modified Monash (MM) fact sheet for category MM 7 covers very remote communities. -

Life Saving Drugs Program (LSDP) Expert Panel meeting agenda – 6 March 2025
Life Saving Drugs Program (LSDP) Expert Panel (the panel) agenda for the 17th meeting on 6 March 2025. -

Avalglucosidase alfa Terms of Reference and Protocol Questions
This report outlines the Avalglucosidase alfa 24 Month Review Terms of Reference and Protocol Questions -

Life Saving Drugs Program (LSDP) Expert Panel meeting agenda – 13 December 2024
Life Saving Drugs Program (LSDP) Expert Panel (the panel) agenda for the 17th meeting on 13 December 2024. -

Life Saving Drugs Program – Hereditary tyrosinaemia (type 1) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for hereditary tyrosinaemia (type 1) for the first time, or after a break. -

Life Saving Drugs Program – Pompe disease – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for Pompe disease for the first time, or after a break. -

Life Saving Drugs Program – Mucopolysaccharidosis type I (MPS I) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for MPS I for the first time, or after a break. -

Life Saving Drugs Program – Mucopolysaccharidosis type II (MPS II) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for MPS II for the first time, or after a break. -

Life Saving Drugs Program – Mucopolysaccharidosis type IVA (MPS IVA) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for MPS IVA for the first time, or after a break. -

Life Saving Drugs Program – Mucopolysaccharidosis type VI (MPS VI) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for MPS VI for the first time, or after a break. -
Life Saving Drugs Program – Gaucher disease (type 1) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for Gaucher disease. -

Life Saving Drugs Program – Hereditary tyrosinaemia (type 1) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for hereditary tyrosinaemia (type 1). -
Life Saving Drugs Program – Late infantile Batten disease (CLN2 disease) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for Batten disease. -
Life Saving Drugs Program – Pompe disease – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for Pompe disease. -
Life Saving Drugs Program – Mucopolysaccharidosis type I (MPS I) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for MPS I. -
Life Saving Drugs Program – Mucopolysaccharidosis type II (MPS II) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for MPS II. -
Life Saving Drugs Program – Mucopolysaccharidosis type IVA (MPS IVA) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for MPS IVA.