Filter results
You can narrow down the results using the filters
Audience
Topics
Our work
Year
23 results
-

NRAS Complexity Review Update - Completion of Phase 4 consultation
The targeted consultation phase of the Review has now concluded. The Final Report is now being drafted and is expected to be delivered to Health Ministers in July 2025 -
National Clinical Quality Registry Program – Participating sites
This map shows which hospitals and health services are currently participating in the clinical quality registries (CQRs) funded under the National Clinical Quality Registry Program. -
National Clinical Quality Registry Program – Best practice materials
This collection contains a suite of best practice guides developed under the National Clinical Quality Registry Program. -
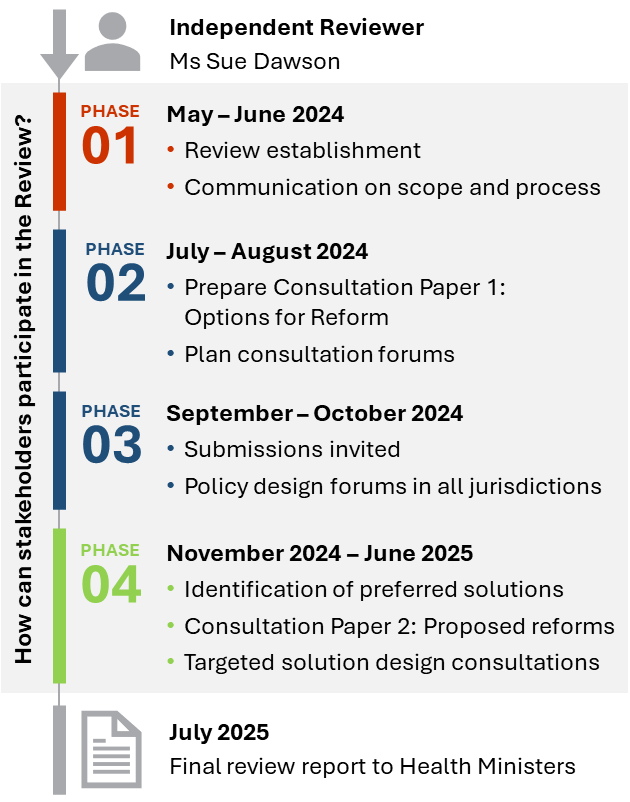
Independent review of complexity in the National Registration and Accreditation Scheme – Project Phases
This diagram outlines the 4 project phases of the Independent Review of Complexity in the National Registration and Accreditation Scheme. Phase 1 commenced in May 2024 with phase 4 to conclude in June 2025. A final Review report is due to Health Ministers in July 2025. -

Consultation Paper 2: Consultation Outcomes and Reform Directions
This Consultation Paper presents a granular exploration of the purpose, nature, and features of a suite of potential reforms, with tangible actions that could be taken to progress those proposed. -

NRAS Complexity review update – Release of Consultation Paper 2
Consultation Paper 2 follows an open public submissions process and wide-reaching consultation conducted during September – November 2024. -

Good Clinical Quality Registry Practice Guide
The guide provides best practice training for hospital staff and clinicians as they begin participating in national Clinical Quality Registries. -

A guide for registry-based trials – National Clinical Quality Registry Program
This guide provides information for Clinical Quality Registry operators and researchers on how to conduct registry-based trials. This guide also offers practical tools and examples to support implementation. -

Update – Extended timeframe for release of Consultation Paper 2
This update provides information on the progress of Consultation Paper 2 and upcoming engagements with stakeholders. -

Health Ministers announce National Board appointments
Health Ministers are very pleased to announce new appointments and reappointments of Chairs, practitioner members and community members to fill vacancies arising across 13 National Boards regulating health professions under the National Registration and Accreditation Scheme (the National Scheme) -

NRAS Complexity Review Update – Completion of phase 3
The Review Team has appreciated the input and feedback on Consultation Paper 1, received in written submissions and through the policy design forums that have been held nationwide. -
-

Consultation Paper 1: Review of complexity in the National Registration and Accreditation Scheme
This Consultation Paper presents evidence collected to date. It identifies the themes that have emerged, the associated issues and challenges, and the potential reform directions. -

National Clinical Quality Registry Program – Communique, August 2024
This communique provides an update on the National Clinical Quality Registry Program in August 2024. It covers upcoming funding opportunities and other news and events. -
National Clinical Quality Registry Program – Communiques
This collection contains communiques that provide updates on the National Clinical Quality Registry Program. -

NRAS Complexity Review update – 18 June 2024
Sue Dawson outlines next steps in the review process and seeks stakeholder advice on how their jurisdiction would prefer to participate and connect throughout the review. -
NRAS Complexity Review updates
This collection contains open letters to stakeholders, updates to proposed consultation forums and progress of the 4 phases for the Independent Review of Complexity in the National Registration and Accreditation Scheme (NRAS). -

Independent review of complexity in the National Registration and Accreditation Scheme – Terms of Reference
These terms of reference outline the scope of the independent review into the complexity of the National Registration and Accreditation Scheme. -

National Clinical Quality Registry Program – Communique, March 2024
This communique provides an update on the National Clinical Quality Registry Program in March 2024. It covers upcoming funding opportunities and other news and events. -

National Clinical Quality Registry Program – Communique, August 2023
This communique provides an update on the National Clinical Quality Registry Program in August 2023. It covers upcoming funding opportunities and other news and events. -

Clinical quality registries – Four key benefits for your health
This publication covers what clinical quality registries do and how they help improve people's health. -

A National Strategy for Clinical Quality Registries and Virtual Registries 2020–2030
This strategy is a roadmap for how the Australian health system will collect and use clinical data to improve patient care. -

Draft national clinical quality registry strategy – Consultation summary report
This report is a summary of the feedback we received during the consultation process in 2019. It highlights key themes that informed the final strategy.