Filter results
You can narrow down the results using the filters
Audience
Topics
Our work
Year
179 results
-

Life Saving Drugs Program (LSDP) Expert Panel meeting agenda – 25 July 2025
Life Saving Drugs Program (LSDP) Expert Panel (the panel) agenda for the 17th meeting on 25 July 2025. -
 26:47
26:47General Practice in Aged Care Incentive – Vodcast 1 – Enabling high quality primary care
The General Practice in Aged Care Incentive will make it easier for older people living in residential aged care homes to receive regular visits and care planning services from their responsible GP and practice. -
 24:01
24:01General Practice in Aged Care Incentive – Vodcast 2 – Enabling team-based care
The General Practice in Aged Care Incentive will make it easier for older people living in residential aged care homes to receive regular visits and care planning services from their responsible GP and practice. -
 11:58
11:58General Practice in Aged Care Incentive – Vodcast 3 – Variations in practice
The General Practice in Aged Care Incentive will make it easier for older people living in residential aged care homes to receive regular visits and care planning services from their responsible GP and practice. -
 22:25
22:25General Practice in Aged Care Incentive – Vodcast 4 – Facilitators and enablers
The General Practice in Aged Care Incentive will make it easier for older people living in residential aged care homes to receive regular visits and care planning services from their responsible GP and practice. -
General Practice in Aged Care Incentive – Vodcasts
Find vodcast video's for the General Practice in Aged Care Incentive. -

Life Saving Drugs Program (LSDP) Expert Panel meeting agenda – 6 March 2025
Life Saving Drugs Program (LSDP) Expert Panel (the panel) agenda for the 17th meeting on 6 March 2025. -

MyMedicare Program Guidelines
The MyMedicare Program Guidelines (the guidelines) provide clear guidance for practices, General Practitioners (GPs) and patients who wish to voluntarily register with the MyMedicare Program (the program). The guidelines also set out the program’s requirements, benefits and dispute processes. -

General Practice in Aged Care Incentive program guidelines 2024
The guidelines provide advice on the service requirements, payments, assessments, and appeals process for the General Practice Aged Care Incentive. -

MyMedicare Terms and Conditions
The MyMedicare Terms and Conditions outline what you are agreeing to when you register in MyMedicare. -
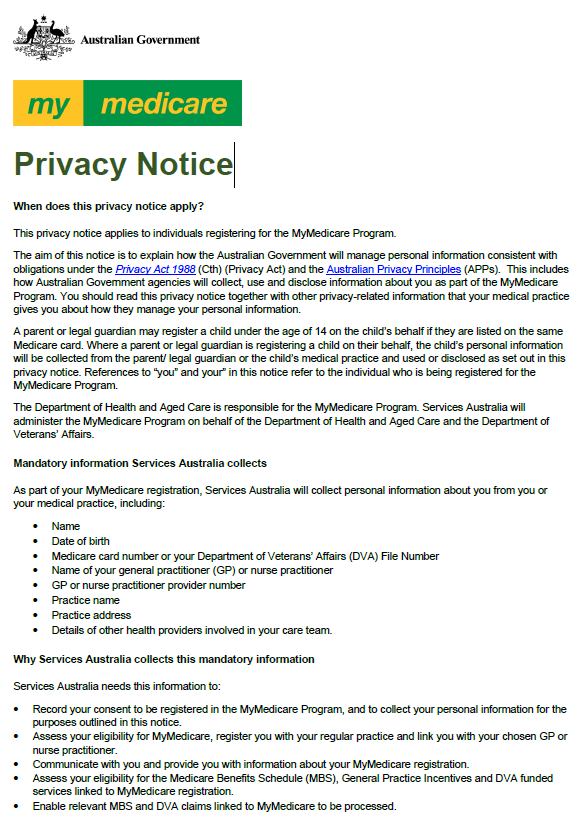
MyMedicare privacy notice
The MyMedicare privacy notice outlines how a patient’s personal information will be collected, used and shared/disclosed when a patient registers for MyMedicare by completing the MyMedicare Patient Registration Form or using Medicare online services. -

Avalglucosidase alfa Terms of Reference and Protocol Questions
This report outlines the Avalglucosidase alfa 24 Month Review Terms of Reference and Protocol Questions -

Life Saving Drugs Program (LSDP) Expert Panel meeting agenda – 13 December 2024
Life Saving Drugs Program (LSDP) Expert Panel (the panel) agenda for the 17th meeting on 13 December 2024. -

Life Saving Drugs Program – Hereditary tyrosinaemia (type 1) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for hereditary tyrosinaemia (type 1) for the first time, or after a break. -

Life Saving Drugs Program – Pompe disease – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for Pompe disease for the first time, or after a break. -

Life Saving Drugs Program – Mucopolysaccharidosis type I (MPS I) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for MPS I for the first time, or after a break. -

Life Saving Drugs Program – Mucopolysaccharidosis type II (MPS II) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for MPS II for the first time, or after a break. -

Life Saving Drugs Program – Mucopolysaccharidosis type IVA (MPS IVA) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for MPS IVA for the first time, or after a break. -

Life Saving Drugs Program – Mucopolysaccharidosis type VI (MPS VI) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for MPS VI for the first time, or after a break. -
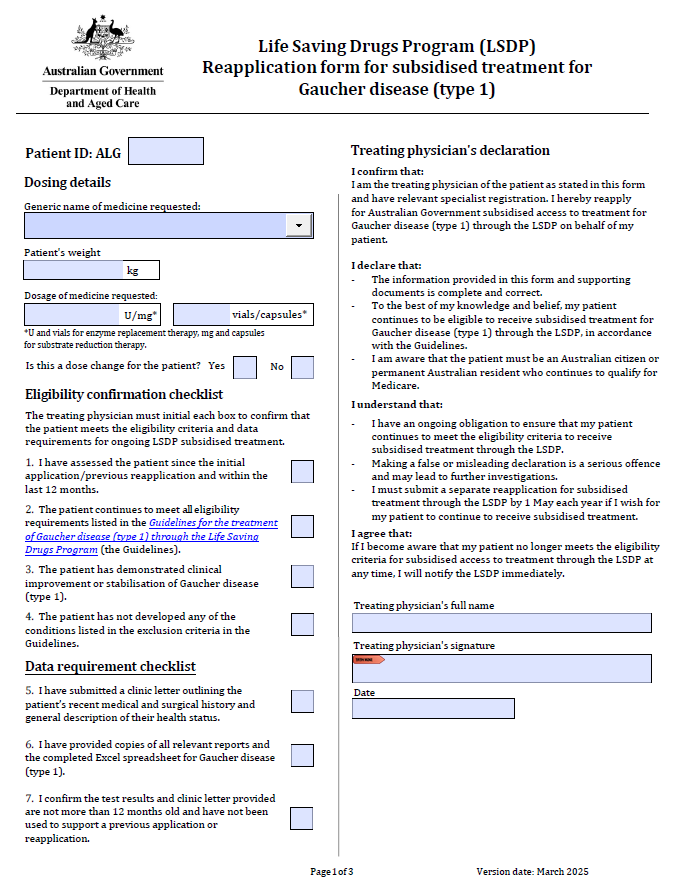
Life Saving Drugs Program – Gaucher disease (type 1) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for Gaucher disease. -

Life Saving Drugs Program – Hereditary tyrosinaemia (type 1) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for hereditary tyrosinaemia (type 1). -
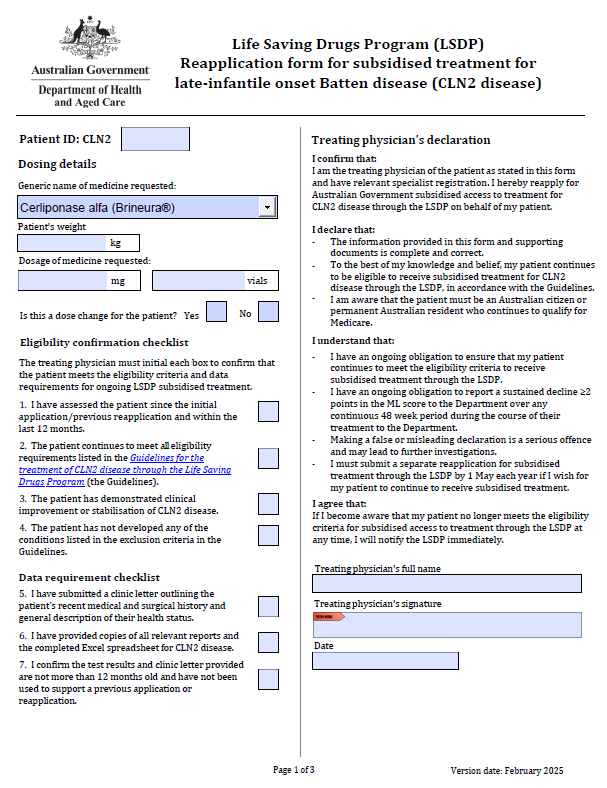
Life Saving Drugs Program – Late infantile Batten disease (CLN2 disease) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for Batten disease. -
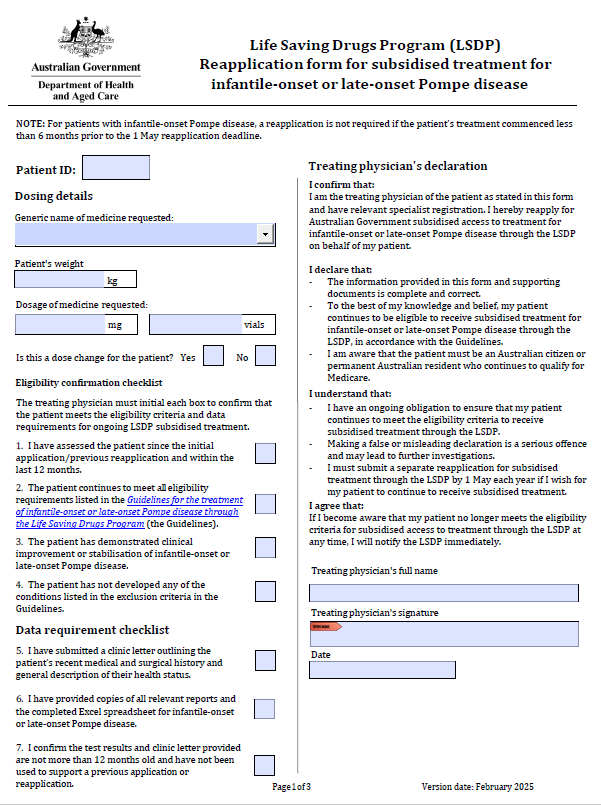
Life Saving Drugs Program – Pompe disease – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for Pompe disease. -
Life Saving Drugs Program – Mucopolysaccharidosis type I (MPS I) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for MPS I. -
Life Saving Drugs Program – Mucopolysaccharidosis type II (MPS II) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for MPS II.

