Filter results
You can narrow down the results using the filters
Audience
Topics
Our work
Diseases
Year
601 results
-

Apply to become a Medicare Benefits Schedule Review Advisory Committee (MRAC) member
This document contains the process to apply for MRAC membership. -

Digital Transformation Sector Partners slide presentation – 3 April 2025
This document summarises the discussion and outcomes of the Digital Transformation Sector Partners meeting on 3 April 2025 -

Digital Transformation Sector Partners meeting summary – 3 April 2025
This document summarises the discussion and outcomes of the Digital Transformation Sector Partners meeting on 2025. -

Digital Transformation Sector Partners slide presentation – 17 April 2025
This document summarises the discussion and outcomes of the Digital Transformation Sector Partners meeting on 17 April 2025. -

Digital Transformation Sector Partners meeting summary – 17 April 2025
This document summarises the discussion and outcomes of the Digital Transformation Sector Partners meeting on 2025. -

Digital Transformation Sector Partners slide presentation – 1 May 2025
This document summarises the discussion and outcomes of the Digital Transformation Sector Partners meeting on 1 May 2025 -

Digital Transformation Sector Partners slide presentation – 15 May 2025
This document summarises the discussion and outcomes of the Digital Transformation Sector Partners meeting on 15 May 2025 -

Digital Transformation Sector Partners meeting summary – 1 May 2025
This document summarises the discussion and outcomes of the Digital Transformation Sector Partners meeting on 2025. -

Digital Transformation Sector Partners meeting summary – 15 May 2025
This document summarises the discussion and outcomes of the Digital Transformation Sector Partners meeting on 2025. -

Draft Cost Recovery Implementation Statement – Administration of the Prescribed List of Medical Devices and Human Tissue Products
This draft cost recovery implementation statement describes how the Department of Health and Aged Care recovers the costs of administering the Prescribed List from 1 July 2025 to 30 June 2026. -

HTA Review Implementation Advisory Group communique – 8 May 2025
This communique summarises the meeting of the Health Technology Assessment (HTA) Review Implementation Advisory Group held on 8 May 2025. -
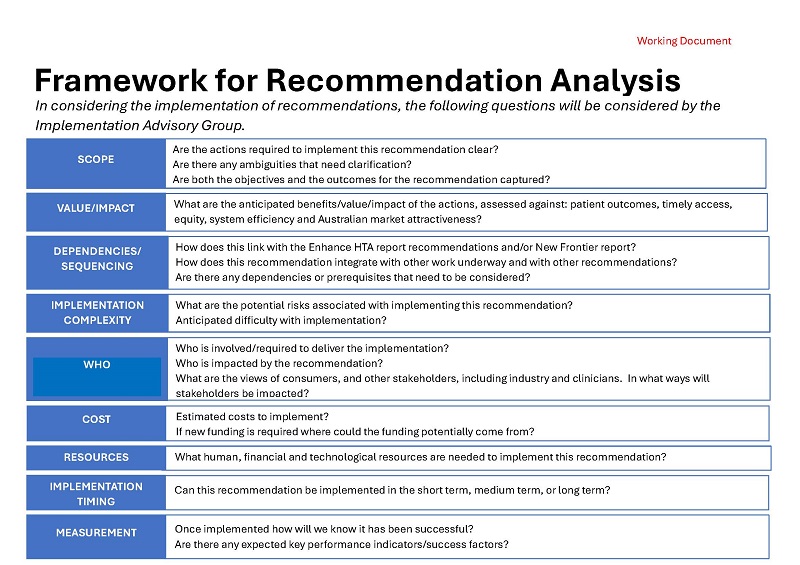
HTA Review Implementation Advisory Group communique – Framework for Recommendation Analysis
This framework is used by Health Technology Assessment (HTA) Review Implementation Advisory Group members to consider the HTA Review's recommendations. -

National Health and Medical Research Strategy Issues Paper – April 2025
A summary of issues raised by the health and medical research sector during consultations on the development of a National Health and Medical Research Strategy. -

Consumer webinar: Health Technology Assessment Review Implementation Advisory Group (IAG) – progress update
This communique summarises the meeting of the Health Technology Assessment (HTA) Review Implementation Advisory Group held on 21 March 2025. -

HTA Review Implementation Advisory Group communique – 10 April 2025
This communique summarises the meeting of the Health Technology Assessment (HTA) Review Implementation Advisory Group held on 10 April 2025. -

HTA Review IAG Consumer Webinar, March 2025 – Chair Presentation
The HTA Review IAG Chair provided a presentation to webinar attendees on the HTA Review process and the IAG’s role. -

HTACCC communique – 19 February 2025
The Health Technology Assessment Consumer Consultative Committee (HTACCC) met on 19 February 2025 for its 28th formal meeting. This document provides a record of the meeting. -

Medicare Benefits Schedule Review Advisory Committee – Terms of reference
This document contains the terms of reference for the Medicare Benefits Schedule (MBS) Review Advisory Committee (MRAC). -

MBS Review Advisory Committee communique – March 2025
This communique is published following each meeting of the MBS Review Advisory Committee (MRAC). It summarises the meeting outcomes, progress on current reviews and advice about upcoming reviews. -

MRFF Traumatic Brain Injury Mission Roadmap – September 2021
This Roadmap outlines the vision, goals and priorities for the Medical Research Future Fund’s (MRFF) Traumatic Brain Injury Mission between September 2021 and March 2025. -

MRFF Traumatic Brain Injury Mission Implementation Plan – September 2021
This plan supported the implementation of the Medical Research Future Fund’s (MRFF) Traumatic Brain Injury Mission Roadmap between September 2021 and March 2025. -

MRFF Traumatic Brain Injury Mission International Review of the Roadmap and Implementation Plan – February 2025
Read the outcomes of the Medical Research Future Fund (MRFF) Traumatic Brain Injury Mission International Review Panel meeting on 6 February 2025. -

MRFF Traumatic Brain Injury Mission Roadmap and Implementation Plan National Consultation Report – March 2025
This report summarises the consultation on the strategic documents for the Traumatic Brain Injury Mission between 8 January 2025 and 10 February 2025. The Traumatic Brain Injury Mission is a Medical Research Future Fund (MRFF) initiative. -
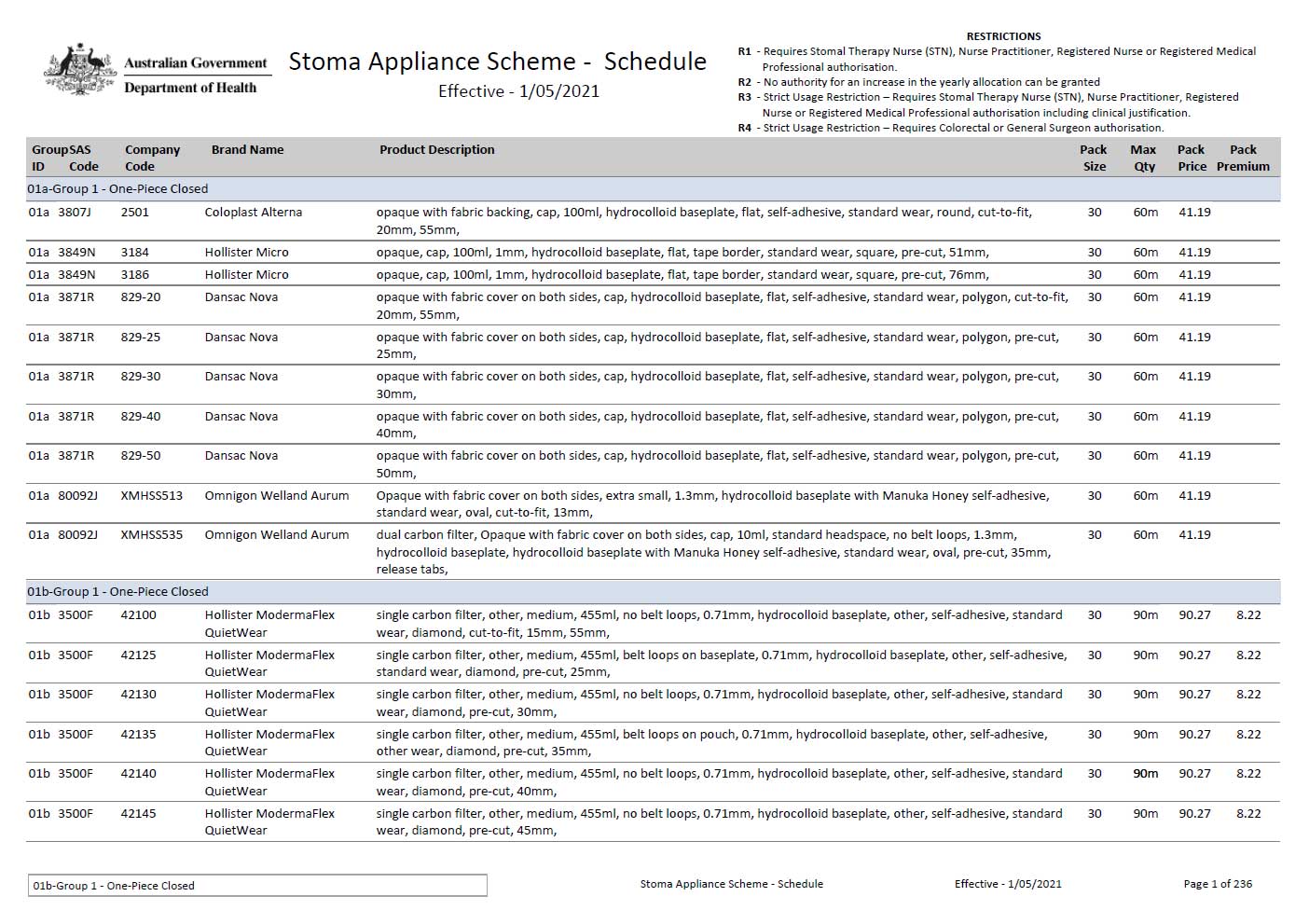
Stoma Appliance Scheme Schedule for download
The Stoma Appliance Scheme Schedule lists all the stoma-related products and appliances that we subsidise under the scheme. You can download the full list in PDF or Excel. -

HTA Review Implementation Advisory Group communique – 11 March 2025
This communique summarises the meeting of the Health Technology Assessment (HTA) Review Implementation Advisory Group held on 11 March 2025
