Filter results
You can narrow down the results using the filters
Audience
Topics
Our work
Diseases
Year
223 results
-

The Australian Type 2 Diabetes Risk Assessment Tool (AUSDRISK) – PDF version
Type 2 diabetes is a common chronic disease. Many Australians are at risk of developing type 2 diabetes, especially if they have certain risk factors. This tool uses a brief list of questions to assess the risk of a person developing type 2 diabetes in the next 5 years. -

Life Saving Drugs Program (LSDP) Expert Panel meeting agenda – 6 March 2025
Life Saving Drugs Program (LSDP) Expert Panel (the panel) agenda for the 17th meeting on 6 March 2025. -
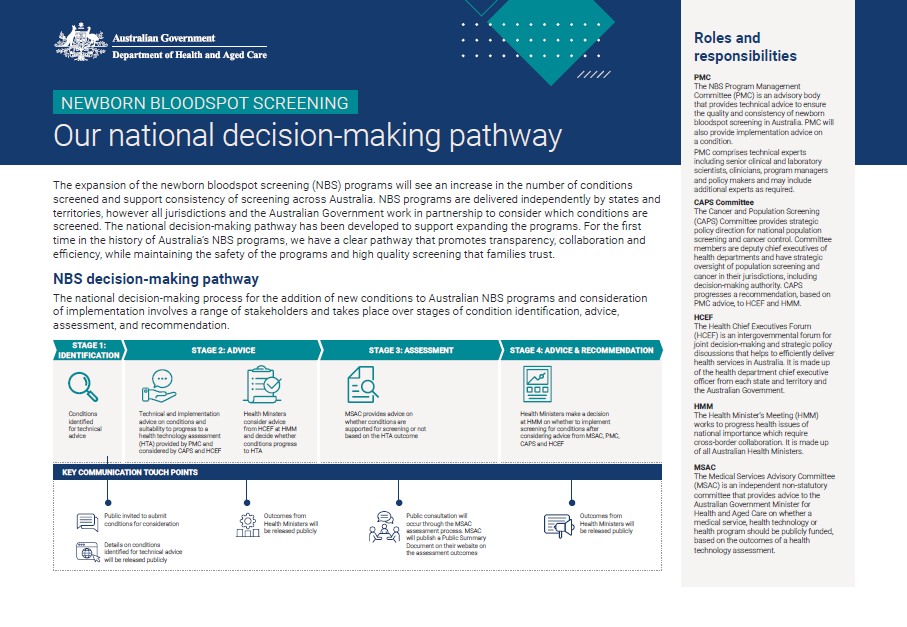
Newborn bloodspot screening (NBS) – Our national decision-making pathway fact sheet
This fact sheet provides information on the national NBS decision-making pathway, including the role of each committee in the process. -
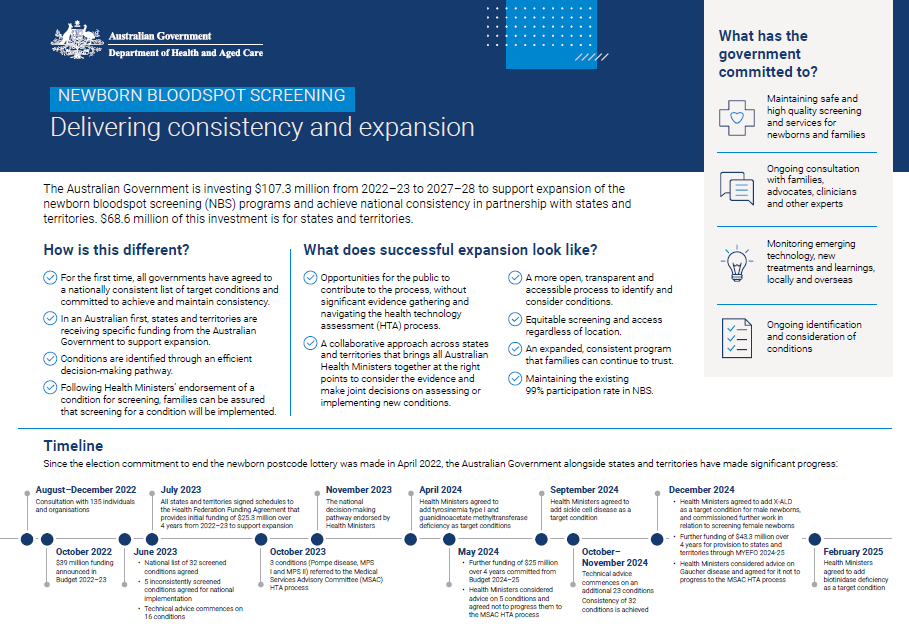
Newborn bloodspot screening (NBS) – Delivering consistency and expansion fact sheet
This fact sheet provides information about what NBS expansion means for families and consumers, expansion progress to date, and upcoming milestones. -

Avalglucosidase alfa Terms of Reference and Protocol Questions
This report outlines the Avalglucosidase alfa 24 Month Review Terms of Reference and Protocol Questions -

Life Saving Drugs Program (LSDP) Expert Panel meeting agenda – 13 December 2024
Life Saving Drugs Program (LSDP) Expert Panel (the panel) agenda for the 17th meeting on 13 December 2024. -

National Occupational Respiratory Disease Registry – Annual Report 2024
The 2024 Annual Report gives an overview of the National Registry and summarises information notified to the National Registry in the first three months of operation and general observations. This Annual Report includes data on mandatory and voluntary notifications, exposure, occupation. -

National Occupational Respiratory Disease Registry postcard
This postcard provides basic information on the National Registry such as the National Registry’s purpose, mandatory and voluntary notifications, and a link to the National Registry website for further information. -

National Occupational Respiratory Disease Registry brochure
This brochure contains key information regarding the National Registry, including types of data collected, purpose of the National Registry, physician obligations, and contact details for the physician help desk. This brochure contains links to the National Registry website and the Physician Portal. -

Australian Government response to the Inquiry into childhood rheumatic diseases – Interim Report
This is the government response to the interim report on Inquiry into childhood rheumatic diseases. -
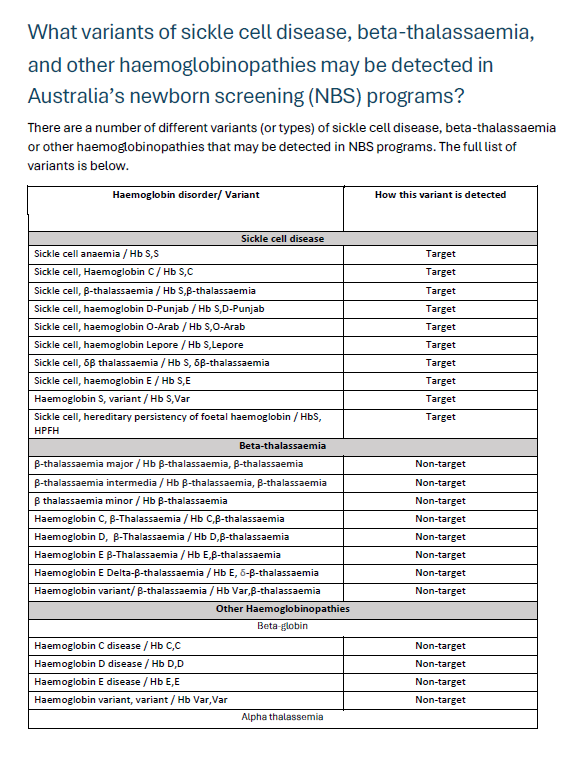
Newborn bloodspot screening variant fact sheet
The list of variants for sickle cell disease, beta thalassemia and other hemoglobinopathies are captured in this resource. -

Life Saving Drugs Program – Hereditary tyrosinaemia (type 1) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for hereditary tyrosinaemia (type 1) for the first time, or after a break. -

Life Saving Drugs Program – Pompe disease – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for Pompe disease for the first time, or after a break. -

Life Saving Drugs Program – Mucopolysaccharidosis type I (MPS I) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for MPS I for the first time, or after a break. -

Life Saving Drugs Program – Mucopolysaccharidosis type II (MPS II) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for MPS II for the first time, or after a break. -

Life Saving Drugs Program – Mucopolysaccharidosis type IVA (MPS IVA) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for MPS IVA for the first time, or after a break. -

Life Saving Drugs Program – Mucopolysaccharidosis type VI (MPS VI) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for MPS VI for the first time, or after a break. -
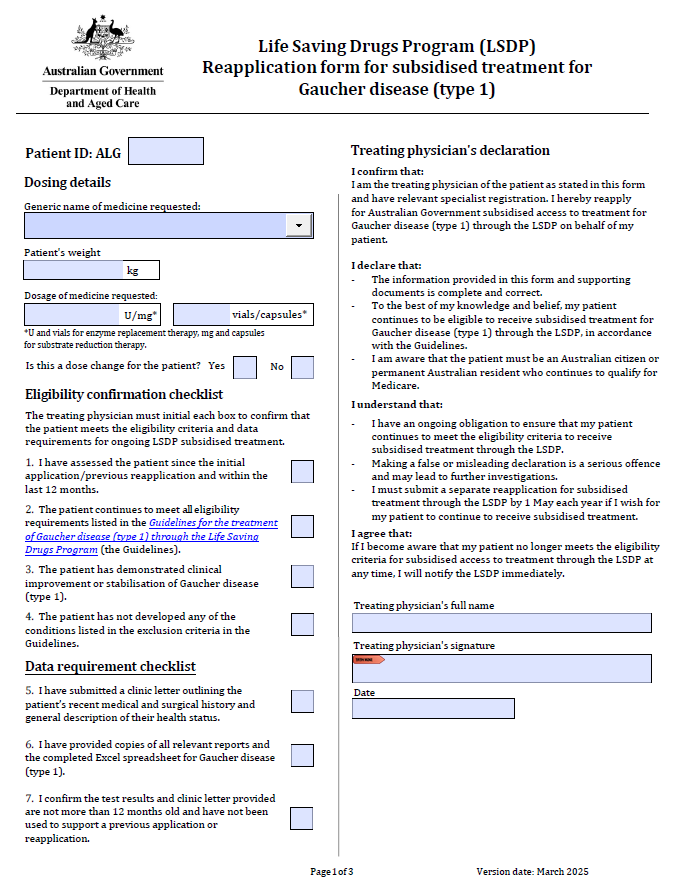
Life Saving Drugs Program – Gaucher disease (type 1) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for Gaucher disease. -

Life Saving Drugs Program – Hereditary tyrosinaemia (type 1) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for hereditary tyrosinaemia (type 1). -
Life Saving Drugs Program – Late infantile Batten disease (CLN2 disease) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for Batten disease. -
Life Saving Drugs Program – Pompe disease – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for Pompe disease. -
Life Saving Drugs Program – Mucopolysaccharidosis type I (MPS I) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for MPS I. -
Life Saving Drugs Program – Mucopolysaccharidosis type II (MPS II) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for MPS II. -
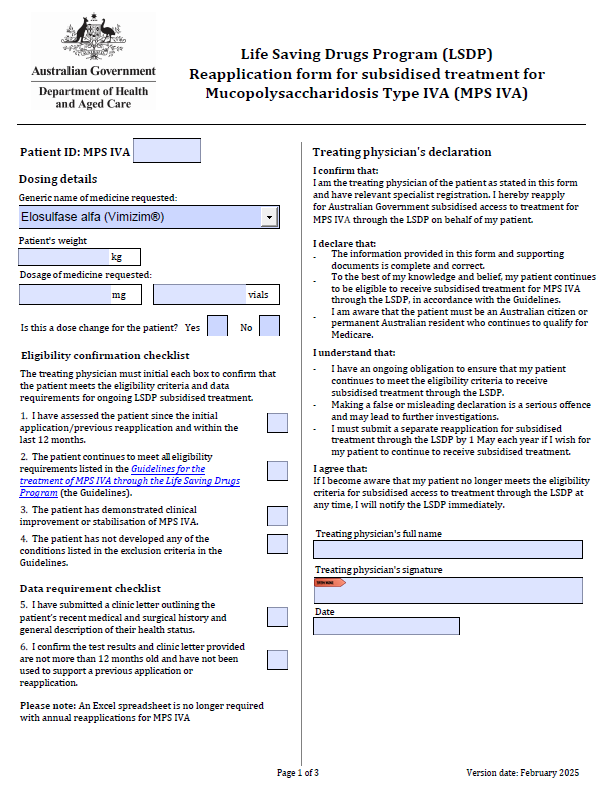
Life Saving Drugs Program – Mucopolysaccharidosis type IVA (MPS IVA) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for MPS IVA. -
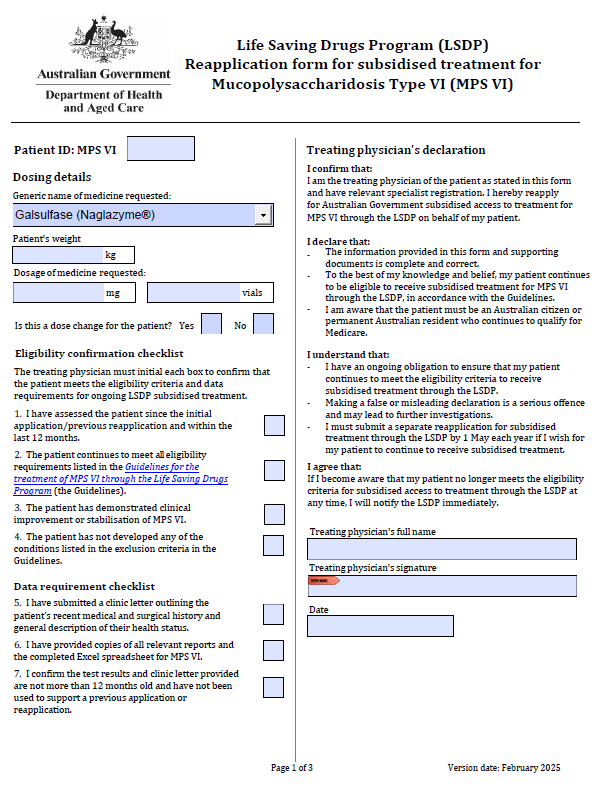
Life Saving Drugs Program – Mucopolysaccharidosis type VI (MPS VI) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for MPS VI.
