Filter results
You can narrow down the results using the filters
Audience
Topics
Our work
Year
12 results
-

Life Saving Drugs Program – Mucopolysaccharidosis type I (MPS I) – Medicines review protocol
This final protocol describes the methodology reviewers will follow to assess use of Life Saving Drugs Program medicines for MPS I. -

Life Saving Drugs Program – Mucopolysaccharidosis type VI (MPS VI) – Medicines review protocol
This final protocol describes the methodology reviewers will follow to assess use of Life Saving Drugs Program medicines for MPS VI. -

Life Saving Drugs Program – Hereditary tyrosinaemia (type 1) – Medicines review protocol
This final protocol describes the methodology reviewers will follow to assess use of Life Saving Drugs Program medicines for hereditary tyrosinaemia (type 1). -

Review of the Quality Use of Medicines Program’s delivery by NPS MedicineWise
This departmental review looked at the effectiveness of the National Prescribing Service, NPS MedicineWise, in delivering the Quality Use of Medicines Program. -

Form of the Electronic Prescription 2019
The Form of the Electronic Prescription 2019 defines the information fields required when a PBS prescriber writes an electronic prescription. -

Electronic Prescriptions Information Technology Requirements Instrument 2019
The Electronic Prescriptions Information Technology Requirements Instrument 2019 details system requirements for participating in electronic prescribing and refers to system obligations to adhere to security, privacy and data usage policies. -

Life Saving Drugs Program – Pompe disease – Medicines review protocol
This final protocol describes the methodology reviewers will follow to assess use of Life Saving Drugs Program medicines for Pompe disease. -

Life Saving Drugs Program – Mucopolysaccharidosis type II (MPS II) – Medicines review protocol
This final protocol describes the methodology reviewers will follow to assess use of Life Saving Drugs Program medicines for MPS II. -

Life Saving Drugs Program – Paroxysmal nocturnal haemoglobinuria – Medicines review protocol
This final protocol describes the methodology reviewers will follow to assess use of Life Saving Drugs Program medicines for paroxysmal nocturnal haemoglobinuria. -

Life Saving Drugs Program – Gaucher disease (type 1) – Medicines review protocol
This final protocol describes the methodology reviewers will follow to assess use of enzyme replacement medicines under the Life Saving Drugs Program medicines for Gaucher disease (type 1). -

Life Saving Drugs Program – Fabry disease – Medicines review protocol
This final protocol describes the methodology reviewers will follow to assess use of Life Saving Drugs Program medicines for Fabry disease. -
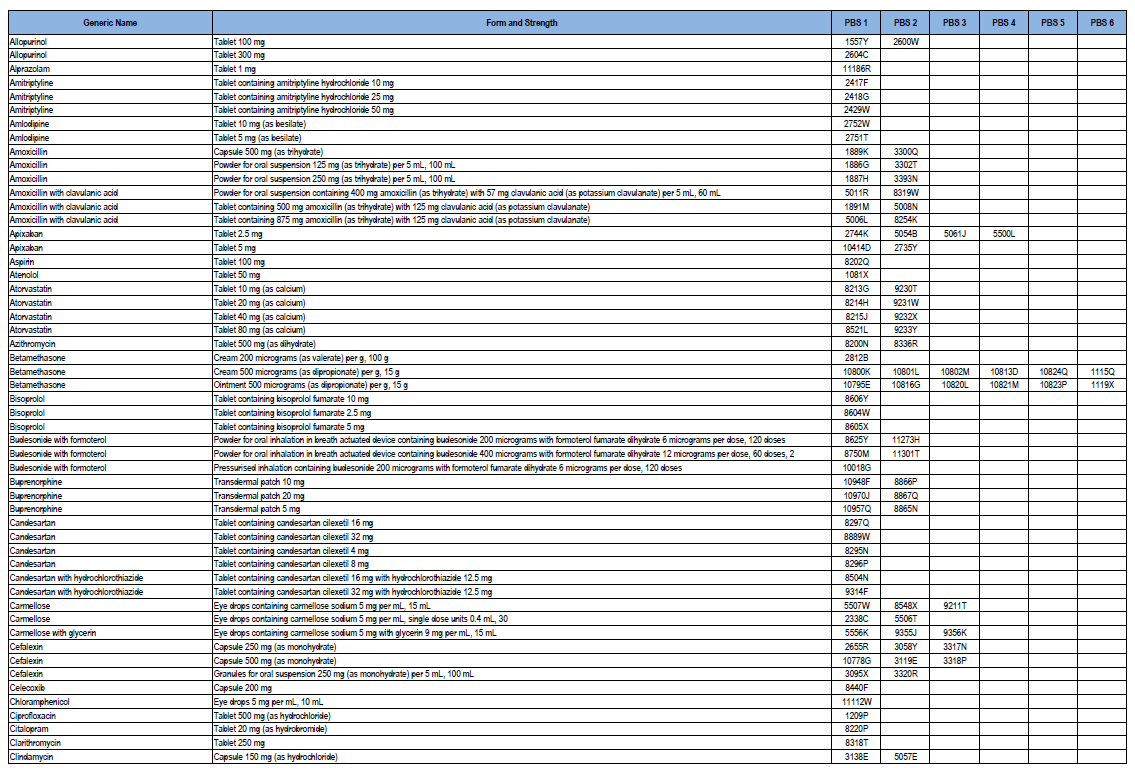
High-volume Pharmaceutical Benefits Scheme (PBS) medicine list 2018–19
This document provides a list of PBS medicines often prescribed, including form and strength, as well as PBS codes, for Community Service Obligation (CSO) for Pharmaceutical Wholesalers funding pool stakeholders for 2018–19.
