Filter results
You can narrow down the results using the filters
Audience
Topics
Our work
Year
46 results
-
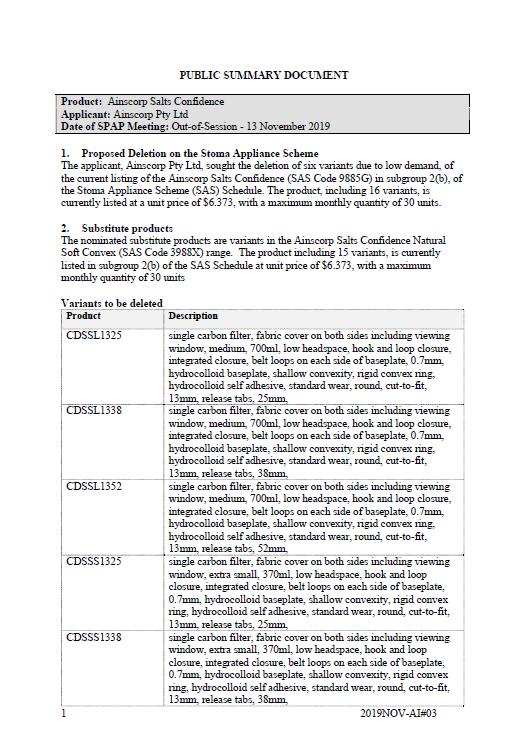
SPAP public summary documents – November 2019 – Ainscorp Salts Confidence one-piece drainable
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to delete 6 variants of the current listing of the Ainscorp Salts Confidence from subgroup 2(b) of the Stoma Appliance Scheme Schedule, due to low demand. -
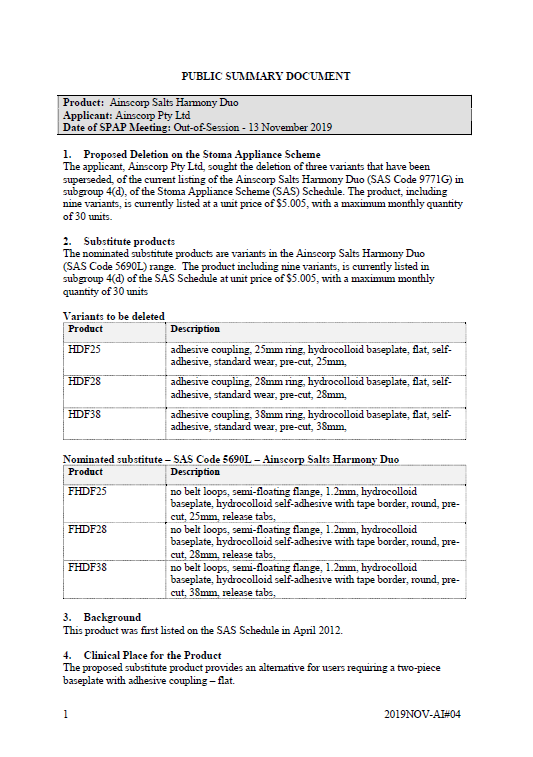
SPAP public summary documents – November 2019 – Ainscorp Salts Harmony Duo two-piece baseplate
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to delete 3 variants that have been superseded from the current listing of the Ainsworth Salts Harmony Duo in subgroup 4(d) of the Stoma Appliance Scheme Schedule. -
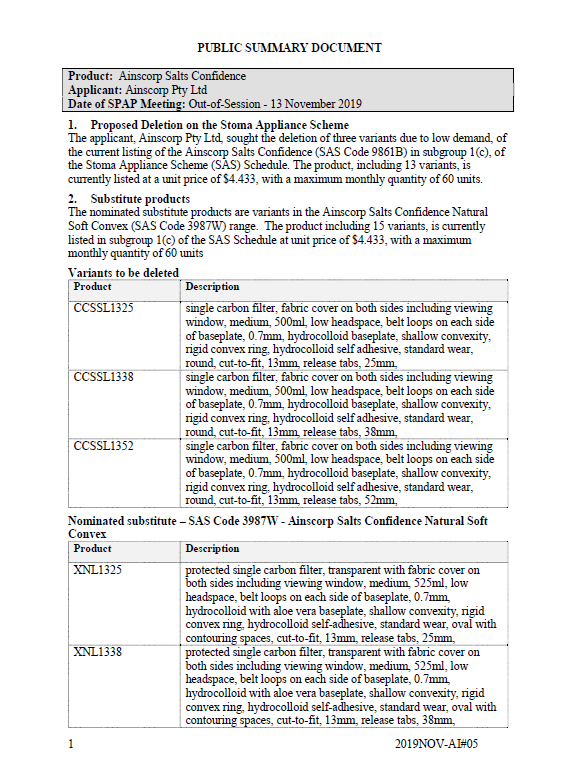
SPAP public summary documents – November 2019 – Ainscorp Salts Confidence one-piece closed
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to delete 3 variants from the current listing of the Ainscorp Salts Confidence in subgroup 1(c) of the Stoma Appliance Scheme Schedule, due to low demand. -
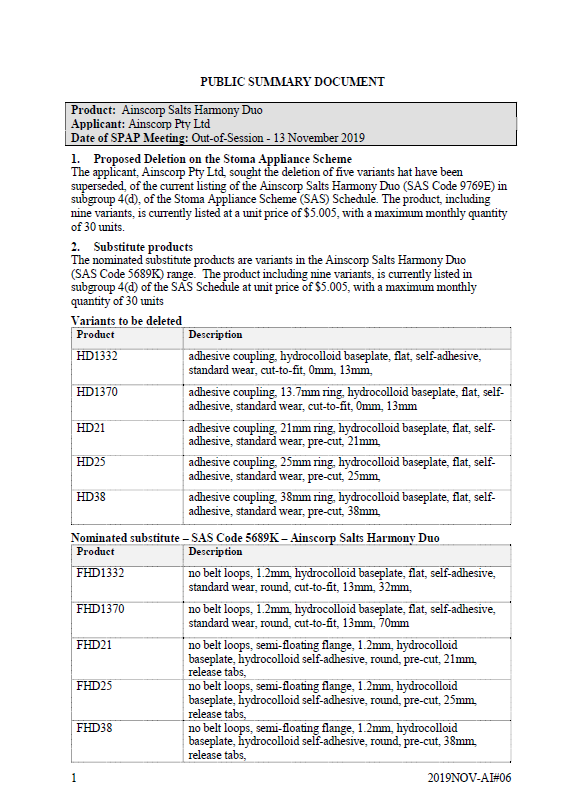
SPAP public summary documents – November 2019 – Ainscorp Salts Harmony Duo two-piece baseplate with adhesive coupling
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to delete 5 variants that have been superseded from the current listing of the Ainscorp Salts Harmony Duo in subgroup 4(d) of the Stoma Appliance Scheme Schedule. -
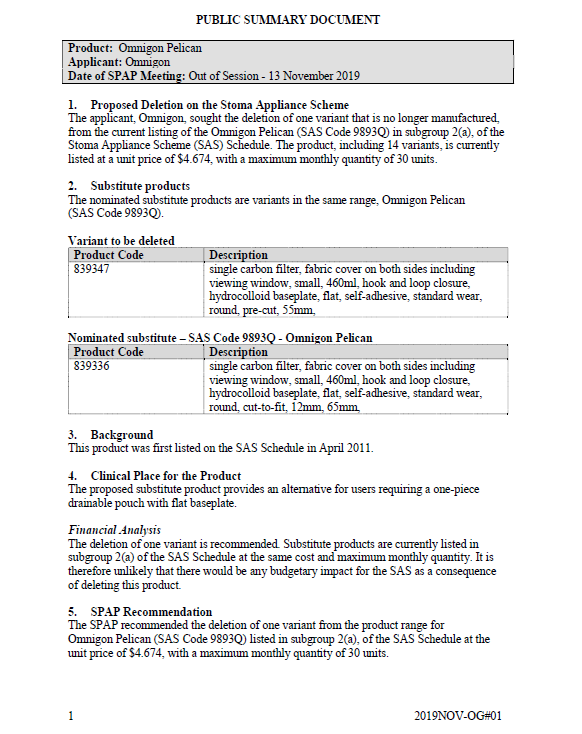
SPAP public summary documents – November 2019 – Omnigon Pelican
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to delete 1 variant that is no longer manufactured from the current listing of the Omnigon Pelican in subgroup 2(a) of the Stoma Appliance Scheme Schedule. -

SPAP public summary documents – October 2019 – review of subgroup 9(h)
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation that all support garments in subgroup 9(h) of the Stoma Appliance Scheme Schedule have 2 restrictions, including a prevention and management plan, added to listed products. -

SPAP public summary documents – October 2019 – Sutherland Medical Suportx Female Hernia Support Girdle
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to list the Sutherland Medical Suportx Female Hernia Support Girdle in subgroup 9(h) of the Stoma Appliance Scheme Schedule. -

SPAP public summary documents – October 2019 – Sutherland Medical Suportx Male Hernia Support Girdle
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to list the Sutherland Medical Suportx Male Hernia Support Girdle in subgroup 9(h) of the Stoma Appliance Scheme Schedule. -

SPAP public summary documents – October 2019 – Sutherland Medical Suportx Breathable Hernia Support Shorts
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to list the Sutherland Medical Suportx Breathable Hernia Support Shorts in subgroup 9(h) of the Stoma Appliance Scheme Schedule. -

SPAP public summary documents – October 2019 – Sutherland Medical Suportx Breathable Hernia Support Briefs
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to list the Sutherland Medical Suportx Breathable Hernia Support Brief in subgroup 9(h) of the Stoma Appliance Scheme Schedule. -

SPAP public summary documents – October 2019 – Sutherland Medical Suportx Shield Belt with Easy Peel Fastening
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to list the Sutherland Medical Suportx Shield Belt with Easy Peel Fastening in subgroup 9(h) of the Stoma Appliance Scheme Schedule. -

SPAP public summary documents – October 2019 – Coloplast Brava Mio Belt
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to change the product description for 4 variants of the Coloplast Brava Mio Belt listed in subgroup 9(b) of the Stoma Appliance Scheme Schedule. -

SPAP public summary documents – October 2019 – Coloplast Brava Protective Seal
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to add 2 variants to the current listing of the Coloplast Brava Protective Seal to subgroup 9(l) of the Stoma Appliance Scheme Schedule. -
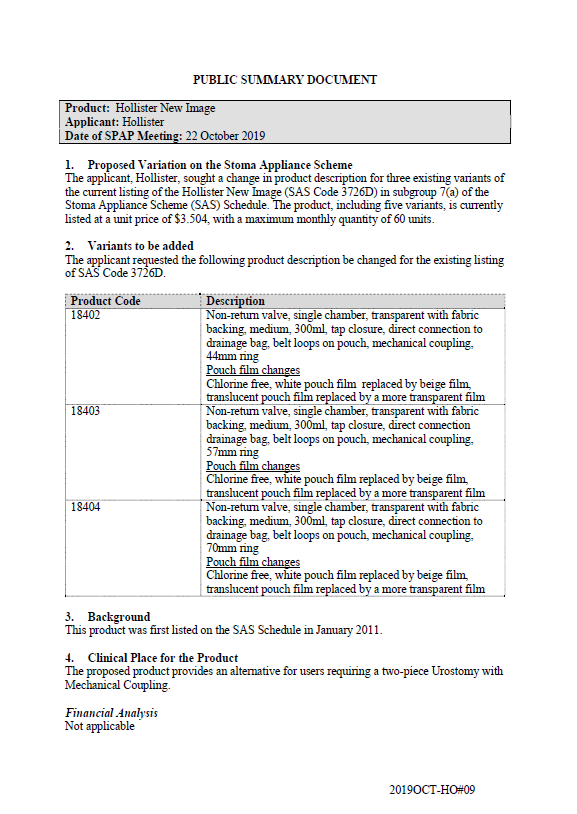
SPAP public summary documents – October 2019 – Hollister New Image Urostomy Pouch
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to change the product description for 3 existing variants of the current listing of Hollister New Image Urostomy Pouch in subgroup7(a) of the Stoma Appliance Scheme Schedule. -
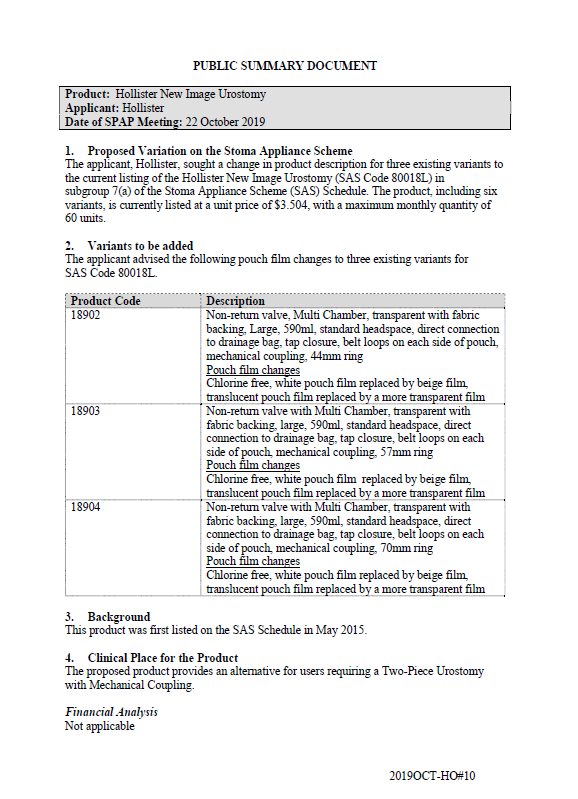
SPAP public summary documents – October 2019 – Hollister New Image Urostomy
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to change the product description for 3 existing variants of the current listing of Hollister New Image Urostomy in subgroup 7(a) of the Stoma Appliance Scheme Schedule. -

SPAP public summary documents – October 2019 – Omnigon Stoma Support Belt
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to add 2 variants to the current listing of Omnigon Stoma Support Belt in subgroup 9(h) of the Stoma Appliance Scheme Schedule. -

SPAP public summary documents – October 2019 – Omnigon Diamond Plus
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to add 2 variants to the current listing of Omnigon Diamon Plus in subgroup 9(h) of the Stoma Appliance Scheme Schedule. -

SPAP public summary documents – October 2019 – Omnigon Diamond Plus neutral
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to add 1 variant to the current listing of Omnigon Diamon Plus in subgroup 9(h) of the Stoma Appliance Scheme Schedule. -

SPAP public summary documents – October 2019 – Omnigon Diamond Plus Men's Support Pants
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to add 1 variant to the current listing of Omnigon Diamon Plus in subgroup 9(h) of the Stoma Appliance Scheme Schedule. -
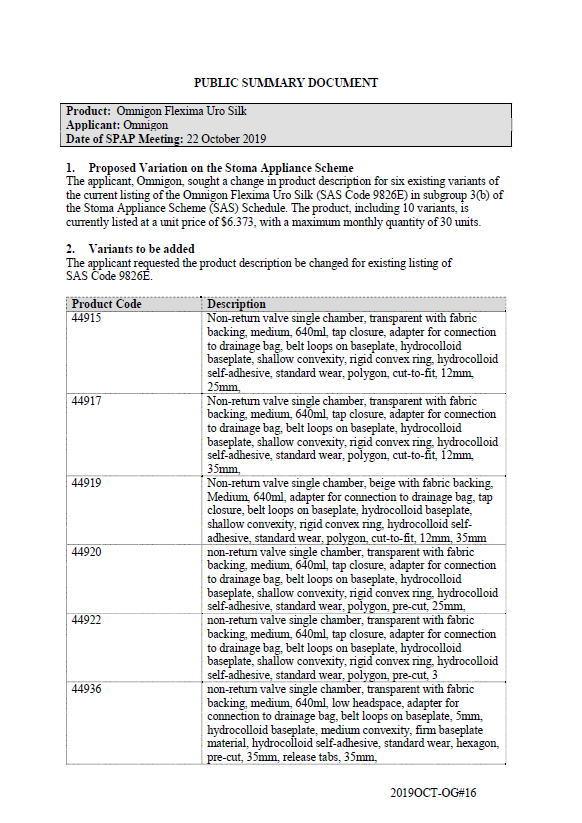
SPAP public summary documents – October 2019 – Omnigon Flexima Uro Silk one-piece
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to change the product description for 6 existing variants of the current listing of the Omnigon Flexima Uro Silk in subgroup 3(b) of the Stoma Appliance Scheme Schedule. -
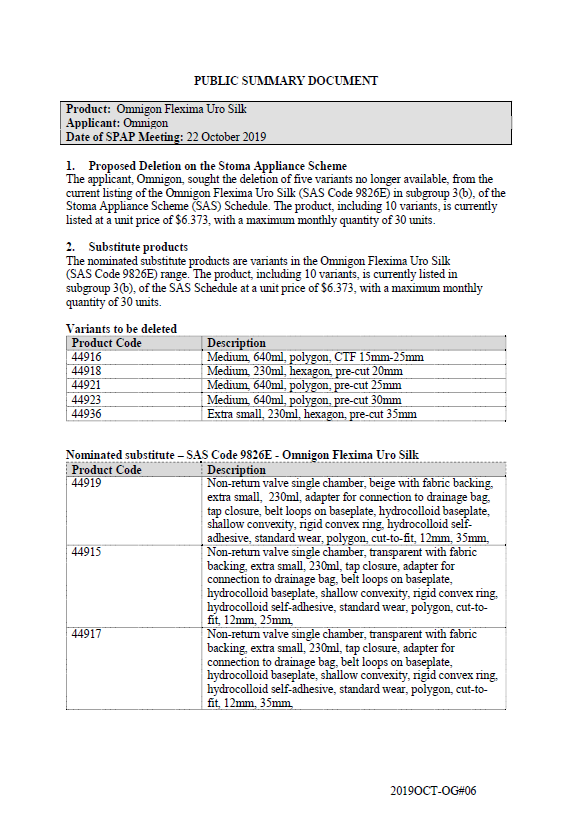
SPAP public summary documents – October 2019 – Omnigon Flexima Uro Silk
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to delete 5 variants that are no longer available from the current listing of Omnigon Flexima Uro Silk in subgroup 3(b) of the Stoma Appliance Scheme Schedule. -
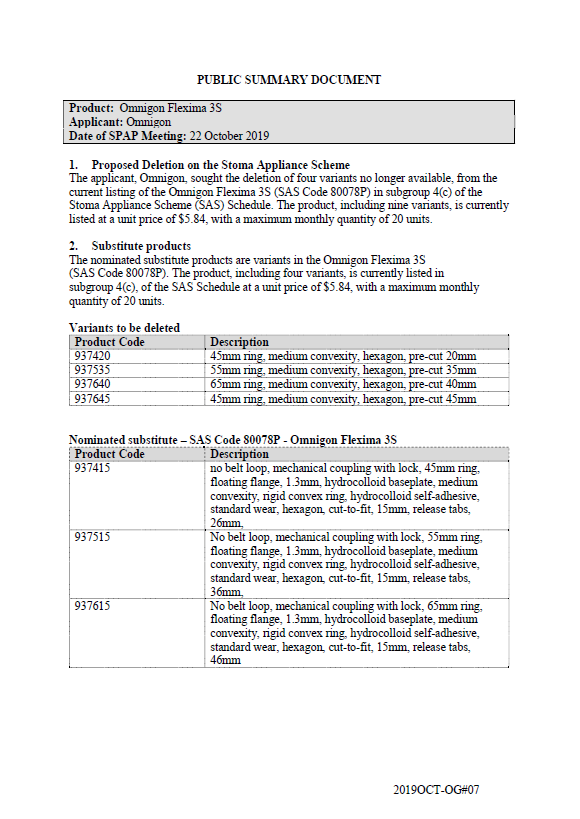
SPAP public summary documents – October 2019 – Omnigon Flexima 3S
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to delete 4 variants that are no longer available from the current listing of Omnigon Flexima 3S in subgroup 4(c) of the Stoma Appliance Scheme Schedule. -

SPAP public summary documents – October 2019 – Omnigon Welland Flair Active
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to delete 2 variants that are no longer available from the current listing of Omnigon Welland Flair Active in subgroup 2(a) of the Stoma Appliance Scheme Schedule. -

SPAP public summary documents – October 2019 – Omnigon Total Control
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to add 3 variants to the current listing of Omnigon Total Control in subgroup 9(h) of the Stoma Appliance Scheme Schedule. -

SPAP public summary documents – October 2019 – Omnigon Support Briefs for Her addition
This public summary document outlines a Stoma Product Assessment Panel (SPAP) recommendation to add 1 variant to the current listing of Omnigon Support Briefs for Her in subgroup 9(h) of the Stoma Appliance Scheme Schedule.
