Filter results
You can narrow down the results using the filters
Audience
Topics
Our work
Year
127 results
-

Life Saving Drugs Program (LSDP) Expert Panel meeting agenda – 6 March 2025
Life Saving Drugs Program (LSDP) Expert Panel (the panel) agenda for the 17th meeting on 6 March 2025. -
Pharmaceutical Benefits Advisory Committee (PBAC) and Medical Services Advisory Committee (MSAC) chairs' letters collection
This collection contains the PBAC & MSAC initial advice and reflections regarding the Health Technology Assessment (HTA) Policy and Methods Review final report, requested by the Minister of Health and Aged Care. -

MSAC chair's letter to the Minister regarding the HTA Review
We established an Implementation Advisory Group to develop a roadmap for sequencing the government’s response to the recommendations. This document contains MSAC chair's response to the Minister regarding the final report of the HTA Review and provided their initial advice and reflections. -

PBAC chair's letter to the Minister regarding the HTA Review
We established an Implementation Advisory Group to develop a roadmap for sequencing the government’s response to the recommendations. This document contains PBAC chair's response to the Minister regarding the final report of the HTA Review and provided their initial advice and reflections. -

Avalglucosidase alfa Terms of Reference and Protocol Questions
This report outlines the Avalglucosidase alfa 24 Month Review Terms of Reference and Protocol Questions -

Life Saving Drugs Program (LSDP) Expert Panel meeting agenda – 13 December 2024
Life Saving Drugs Program (LSDP) Expert Panel (the panel) agenda for the 17th meeting on 13 December 2024. -

Life Saving Drugs Program – Hereditary tyrosinaemia (type 1) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for hereditary tyrosinaemia (type 1) for the first time, or after a break. -

Life Saving Drugs Program – Pompe disease – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for Pompe disease for the first time, or after a break. -

Life Saving Drugs Program – Mucopolysaccharidosis type I (MPS I) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for MPS I for the first time, or after a break. -

Life Saving Drugs Program – Mucopolysaccharidosis type II (MPS II) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for MPS II for the first time, or after a break. -

Life Saving Drugs Program – Mucopolysaccharidosis type IVA (MPS IVA) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for MPS IVA for the first time, or after a break. -

Life Saving Drugs Program – Mucopolysaccharidosis type VI (MPS VI) – Initial application
Treating physicians use this application form to apply for a patient to access LSDP medication for MPS VI for the first time, or after a break. -
Life Saving Drugs Program – Gaucher disease (type 1) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for Gaucher disease. -

Life Saving Drugs Program – Hereditary tyrosinaemia (type 1) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for hereditary tyrosinaemia (type 1). -
Life Saving Drugs Program – Late infantile Batten disease (CLN2 disease) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for Batten disease. -
Life Saving Drugs Program – Pompe disease – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for Pompe disease. -
Life Saving Drugs Program – Mucopolysaccharidosis type I (MPS I) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for MPS I. -
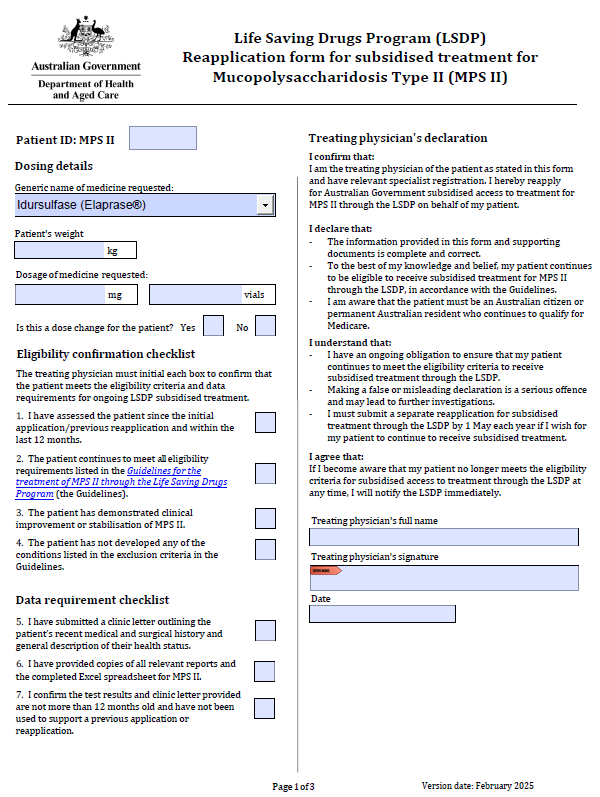
Life Saving Drugs Program – Mucopolysaccharidosis type II (MPS II) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for MPS II. -
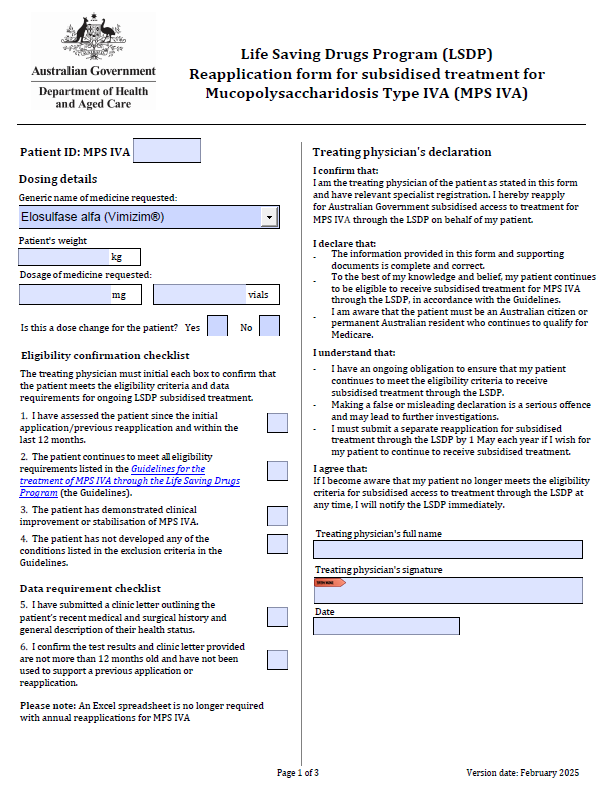
Life Saving Drugs Program – Mucopolysaccharidosis type IVA (MPS IVA) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for MPS IVA. -
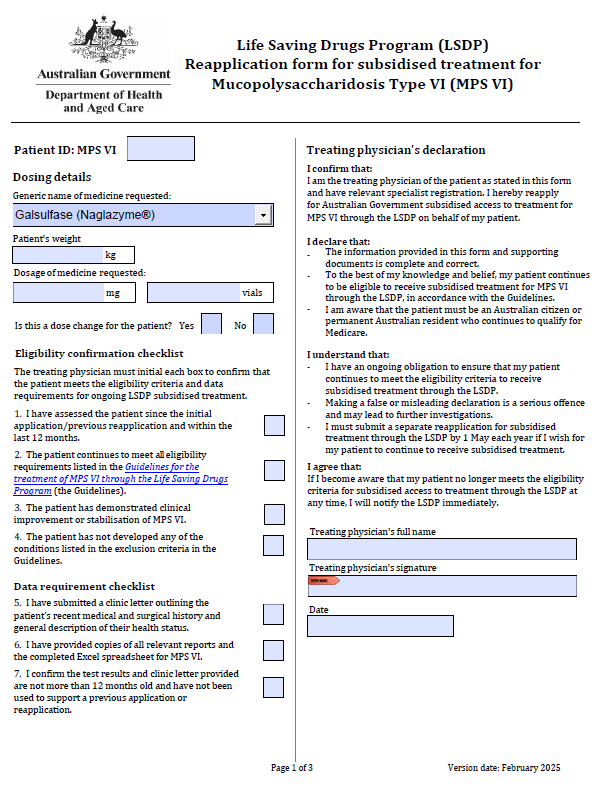
Life Saving Drugs Program – Mucopolysaccharidosis type VI (MPS VI) – Reapplication
Treating physicians use this form to reapply each year for a patient to receive ongoing LSDP medication for MPS VI. -

Life Saving Drugs Program – Gaucher disease (type 1) – Guidelines
These guidelines contain the general, initial and ongoing eligibility requirements to access certain medications for Gaucher disease (type 1) under the Life Saving Drugs Program. -
Life Saving Drugs Program – Hereditary tyrosinaemia (type 1) – Guidelines
These guidelines contain the general, initial and ongoing eligibility requirements to access treatment for hereditary tyrosinaemia (type 1) under the Life Saving Drugs Program. -

Life Saving Drugs Program – Late infantile Batten disease (CLN2)
These guidelines contain the general, initial and ongoing eligibility requirements to access treatment for late-infantile onset Batten disease under the Life Saving Drugs Program. -

Life Saving Drugs Program – Pompe disease – Guidelines
These guidelines contain the general, initial and ongoing eligibility requirements to access treatment for infantile-onset or late-onset Pompe disease under the Life Saving Drugs Program. -

Life Saving Drugs Program – Mucopolysaccharidosis type I (MPS I) – Guidelines
These guidelines contain the general, initial and ongoing eligibility requirements to access treatment for mucopolysaccharidosis type I (MPS I) under the Life Saving Drugs Program.

