Filter results
You can narrow down the results using the filters
Audience
Publication type
Topics
Our work
Diseases
Year
108 results
-

HTA Review Implementation Advisory Group communique – 8 May 2025
This communique summarises the meeting of the Health Technology Assessment (HTA) Review Implementation Advisory Group held on 8 May 2025. -
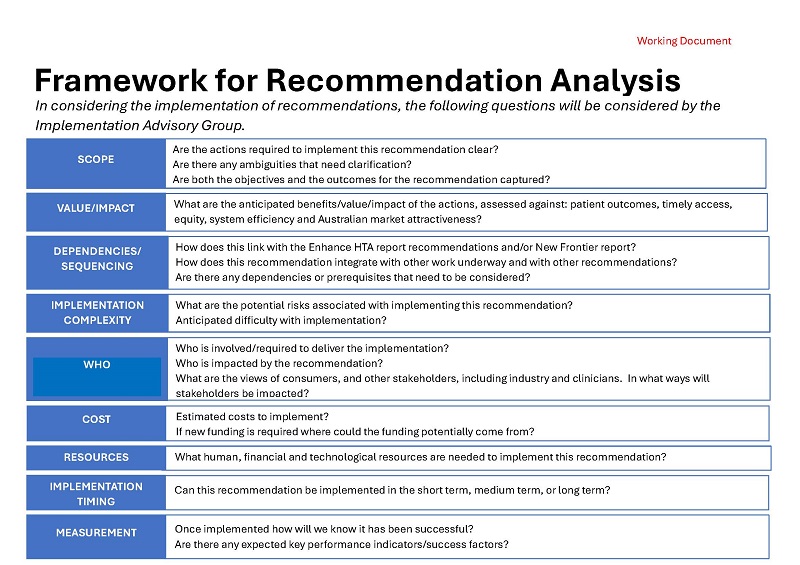
HTA Review Implementation Advisory Group communique – Framework for Recommendation Analysis
This framework is used by Health Technology Assessment (HTA) Review Implementation Advisory Group members to consider the HTA Review's recommendations. -

Consumer webinar: Health Technology Assessment Review Implementation Advisory Group (IAG) – progress update
This communique summarises the meeting of the Health Technology Assessment (HTA) Review Implementation Advisory Group held on 21 March 2025. -

HTA Review Implementation Advisory Group communique – 10 April 2025
This communique summarises the meeting of the Health Technology Assessment (HTA) Review Implementation Advisory Group held on 10 April 2025. -

National syphilis surveillance quarterly report – October to December 2024
This is the latest report in a series of national quarterly syphilis surveillance reports. -

HTA Review Implementation Advisory Group communique – 11 March 2025
This communique summarises the meeting of the Health Technology Assessment (HTA) Review Implementation Advisory Group held on 11 March 2025 -

Minister for Health & Aged Care letter to Professor Andrew Wilson AO, IAG Chair
This letter outlines the Minister for Health & Aged Care’s priority areas for IAG consideration. -

HTA Review Implementation Advisory Group communique – 3 February 2025
This communique summarises the meeting of the Health Technology Assessment (HTA) Review Implementation Advisory Group held on 3 February 2025. -

Health Technology Assessment Review Implementation Advisory Group – Terms of reference
Terms of reference for the Health Technology Assessment Review Implementation Advisory Group. -

National syphilis surveillance quarterly report – July to September 2024
This is the latest report in a series of national quarterly syphilis surveillance reports. -

MSAC chair's letter to the Minister regarding the HTA Review
We established an Implementation Advisory Group to develop a roadmap for sequencing the government’s response to the recommendations. This document contains MSAC chair's response to the Minister regarding the final report of the HTA Review and provided their initial advice and reflections. -

PBAC chair's letter to the Minister regarding the HTA Review
We established an Implementation Advisory Group to develop a roadmap for sequencing the government’s response to the recommendations. This document contains PBAC chair's response to the Minister regarding the final report of the HTA Review and provided their initial advice and reflections. -

National syphilis surveillance quarterly report – April to June 2024
This report provides a quarterly account of progress against the targets and indicators in the National strategic approach for responding to rising rates of syphilis in Australia 2021 and National syphilis surveillance and monitoring plan. -

Health Technology Assessment Policy and Methods Review – Final report
This document is the final report from the Health Technology Assessment Policy and Methods Review. -

Health Technology Assessment Policy and Methods Review – Recommendations summary
This document summarises the recommendations from the Health Technology Assessment Policy and Methods Review report. -

Health Technology Assessment Policy and Methods Review – Full recommendations
This document outlines all of the recommendations from the Health Technology Assessment Policy and Methods Review final report. -

Enhance HTA: An Enhanced Consumer Engagement Process in Australian Health Technology Assessment – A report of recommendations
The Enhanced Consumer Engagement Process was developed to provide recommendations on how to increase patient and consumer evidence and input earlier in the Health Technology Assessment (HTA) process. This report explains the recommendations developed by the Co-Design Working Group. -

National syphilis surveillance quarterly report – January to March 2024
This report provides a quarterly account of progress against the targets and indicators in the National strategic approach for responding to rising rates of syphilis in Australia 2021 and National syphilis surveillance and monitoring plan. -

HTA Policy and Methods Review – Emerging health technologies
This paper examines emerging health technologies. -

HTA Policy and Methods Review – Australian market authorisation, funding and assessment pathways and timelines
This paper examines the Australian market authorisation, funding and assessment pathways and timelines. -

HTA Policy and Methods Review – HTA pathways and processes, clinical evaluation methods and horizon scanning
This paper examines the pathways, processes and steps used in health technology assessment (HTA) internationally. -

HTA Policy and Methods Review – HTA Methods: Economic evaluation
This paper provides an overview of the methods used in economic evaluation as part of HTA processes in Australia and other jurisdictions of interest where HTA is used to support reimbursement for new health technologies. -

HTA Policy and Methods Review – Funding and purchasing decisions and managing uncertainty
This discussion paper examines local and international approaches for funding and purchasing arrangements and the managing uncertainty. -

HTA Policy and Methods Review – Optimising the availability and use of real world data and real world evidence to support health technology assessment
This paper examines real world evidence to support health technology assessment (HTA) in Australia. -

Health Technology Assessment Policy and Methods Review – Reference Committee communique – 1 March 2024 to 2 May 2024
The communique covers the deliberative period of the Health Technology Assessment Policy and Methods Review, leading into the completion of the final report.
