Filter results
You can narrow down the results using the filters
Audience
Topics
Our work
Year
99 results
-

HTA Review Implementation Advisory Group communique – 12 June 2025
This communique summarises the meeting of the Health Technology Assessment (HTA) Review Implementation Advisory Group held on 12 June 2025. -

HTA Review Implementation Advisory Group communique – 8 May 2025
This communique summarises the meeting of the Health Technology Assessment (HTA) Review Implementation Advisory Group held on 8 May 2025. -
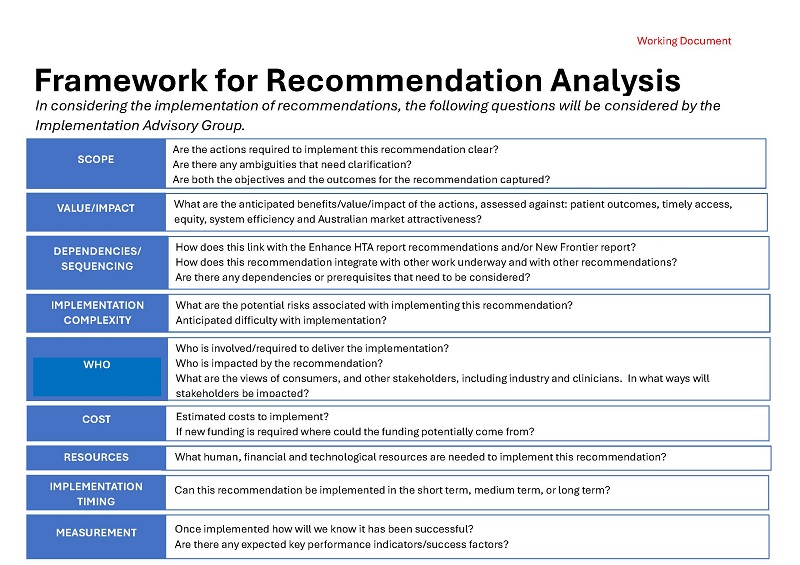
HTA Review Implementation Advisory Group – Framework for Recommendation Analysis
This framework is used by Health Technology Assessment (HTA) Review Implementation Advisory Group members to consider the HTA Review's recommendations. -

Consumer webinar: Health Technology Assessment Review Implementation Advisory Group (IAG) – progress update
This communique summarises the meeting of the Health Technology Assessment (HTA) Review Implementation Advisory Group held on 21 March 2025. -

HTA Review Implementation Advisory Group communique – 10 April 2025
This communique summarises the meeting of the Health Technology Assessment (HTA) Review Implementation Advisory Group held on 10 April 2025. -

Approved Medical Deputising Services (AMDS) program – Application form for a doctor placement
Doctors should use this form when applying for an initial or subsequent to Approved Medical Deputising Services (AMDS) program placement with their chosen Service Provider. -

Approved Medical Deputising Services (AMDS) program – Application form for a Service Provider Deed of Agreement
Medical deputising services should use this form when applying for an initial or subsequent Deed of Agreement. -
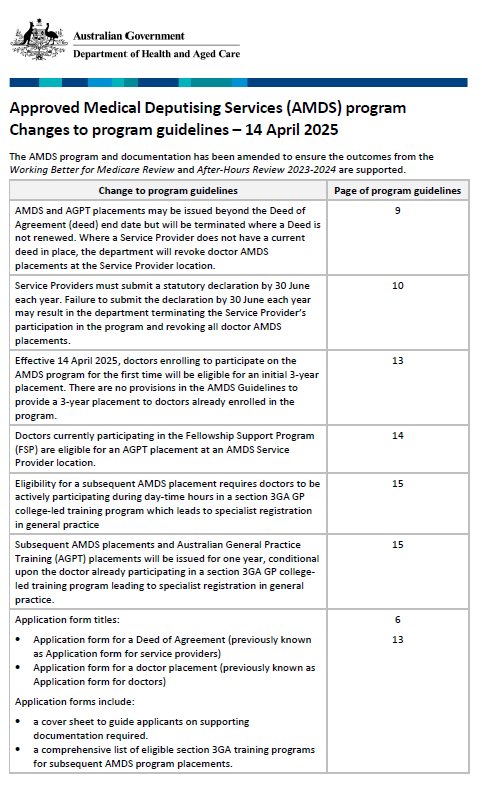
Approved Medical Deputising Services (AMDS) – Changes to program guidelines April 2025
This document outlines what changes we made to the AMDS guidelines in April 2025, as well as the reasons for those changes. -

Approved Medical Deputising Services (AMDS) program guidelines
These guidelines provide information about how the Approved Medical Deputising Services (AMDS) program works, including procedures, responsibilities, eligibility and application processes for both doctors and service providers. -

HTA Review Implementation Advisory Group communique – 11 March 2025
This communique summarises the meeting of the Health Technology Assessment (HTA) Review Implementation Advisory Group held on 11 March 2025 -

Minister for Health & Aged Care letter to Professor Andrew Wilson AO, IAG Chair
This letter outlines the Minister for Health & Aged Care’s priority areas for IAG consideration. -
Health Technology Assessment Review Implementation Advisory Group – Communiques
This collection contains communiques from the Health Technology Assessment (HTA) Review Implementation Advisory Group (IAG). -

HTA Review Implementation Advisory Group communique – 3 February 2025
This communique summarises the meeting of the Health Technology Assessment (HTA) Review Implementation Advisory Group held on 3 February 2025. -

Health Technology Assessment Review Implementation Advisory Group – Terms of reference
Terms of reference for the Health Technology Assessment Review Implementation Advisory Group. -
Pharmaceutical Benefits Advisory Committee (PBAC) and Medical Services Advisory Committee (MSAC) chairs' letters collection
This collection contains the PBAC & MSAC initial advice and reflections regarding the Health Technology Assessment (HTA) Policy and Methods Review final report, requested by the Minister of Health and Aged Care. -

MSAC chair's letter to the Minister regarding the HTA Review
We established an Implementation Advisory Group to develop a roadmap for sequencing the government’s response to the recommendations. This document contains MSAC chair's response to the Minister regarding the final report of the HTA Review and provided their initial advice and reflections. -

PBAC chair's letter to the Minister regarding the HTA Review
We established an Implementation Advisory Group to develop a roadmap for sequencing the government’s response to the recommendations. This document contains PBAC chair's response to the Minister regarding the final report of the HTA Review and provided their initial advice and reflections. -

Enhance HTA: An Enhanced Consumer Engagement Process in Australian Health Technology Assessment – A report of recommendations
The Enhanced Consumer Engagement Process was developed to provide recommendations on how to increase patient and consumer evidence and input earlier in the Health Technology Assessment (HTA) process. This report explains the recommendations developed by the Co-Design Working Group. -

Health Technology Assessment Policy and Methods Review – Final report
This document is the final report from the Health Technology Assessment Policy and Methods Review. -

Health Technology Assessment Policy and Methods Review – Recommendations summary
This document summarises the recommendations from the Health Technology Assessment Policy and Methods Review report. -

Health Technology Assessment Policy and Methods Review – Full recommendations
This document outlines all of the recommendations from the Health Technology Assessment Policy and Methods Review final report. -
HTA Review final report collection
This collection contains 3 main documents related to the Health Technology Assessment (HTA) Policy and Methods Review final report. -

HTA Policy and Methods Review – Australian market authorisation, funding and assessment pathways and timelines
This paper examines the Australian market authorisation, funding and assessment pathways and timelines. -

HTA Policy and Methods Review – Emerging health technologies
This paper examines emerging health technologies. -

HTA Policy and Methods Review – HTA pathways and processes, clinical evaluation methods and horizon scanning
This paper examines the pathways, processes and steps used in health technology assessment (HTA) internationally.



